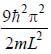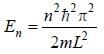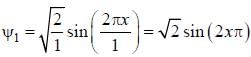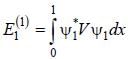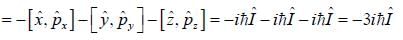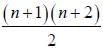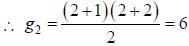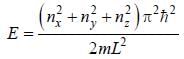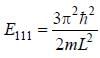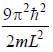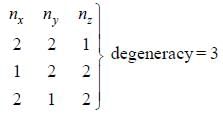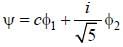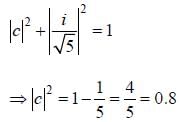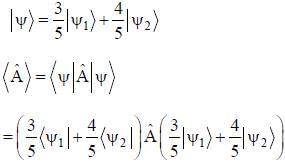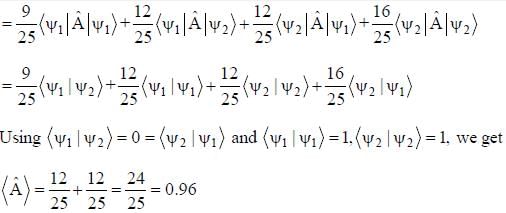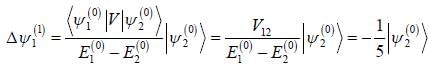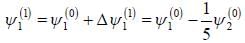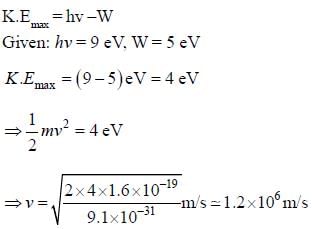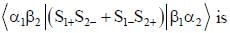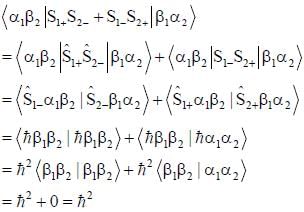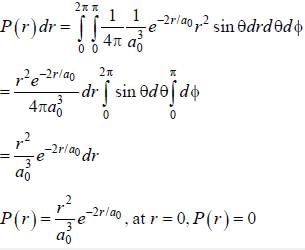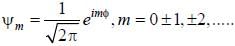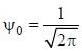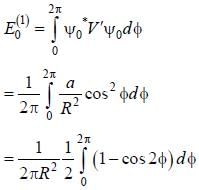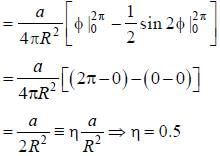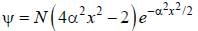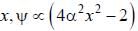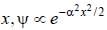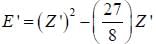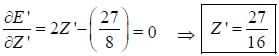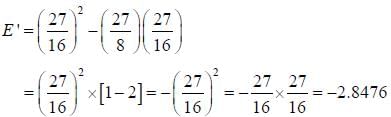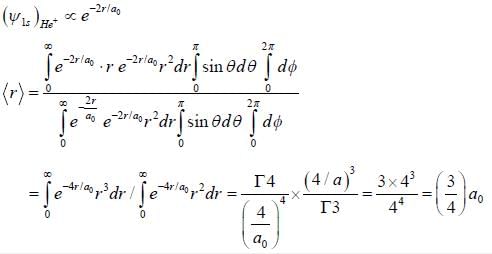Quantum Chemistry - 2 - Chemistry MCQ
20 Questions MCQ Test - Quantum Chemistry - 2
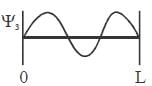
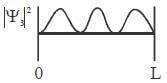
For the energy level 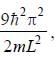 the probability density for a particle of mass m is a one dimensional potential box of width L is given by
the probability density for a particle of mass m is a one dimensional potential box of width L is given by
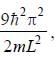 the probability density for a particle of mass m is a one dimensional potential box of width L is given by
the probability density for a particle of mass m is a one dimensional potential box of width L is given byπ-boncl length between C-C atoms. f.i and v is calculated by using the empirical formula,
Rμv = 1.52Å - 0.186 Å Pμv
where Pμv= is π - bond order between μ and v C -atom .
According to HM O theory. delocalization energy o f Benzene is 2|β|. Then T-bond length in Benzene is
Rμv = 1.52Å - 0.186 Å Pμv
where Pμv= is π - bond order between μ and v C -atom .
According to HM O theory. delocalization energy o f Benzene is 2|β|. Then T-bond length in Benzene is
According to Huckel molecular orbital theory, the total energy of hypothetical localized Benzene (C6H6) molecule is
A perturbation 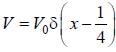 is introduced in a box of width 1 unit, where V0 is ( << 1 ) is a positive constant. The first order energy correction to the first exited state is α V0 . The value of α is_________
is introduced in a box of width 1 unit, where V0 is ( << 1 ) is a positive constant. The first order energy correction to the first exited state is α V0 . The value of α is_________
σx , σy and σz are the Pauli m atrices. The expression is equal to 2σx σy + σy σx is equal to
For linear momentum vector operator 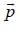 -and position vector operator
-and position vector operator 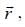 the operator
the operator 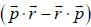 is equivalent to
is equivalent to
The degeneracy of the n = 2 level for a three dimensional isotropic oscillator is ________. (an integer)
The operation of the commutator 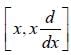 on a function f ( x ) is equal to
on a function f ( x ) is equal to
The degeneracy of a quantum particle in a cubic box having energy three times that of the lowest energy is ________
Consider a normalized molecular orbital 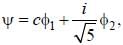 constructed from two different atomic orbitals
constructed from two different atomic orbitals 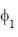 and
and 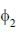 that fonn an orthonormal set. Here i = √-1. The value of |c|2 is ______ (upto one decim al place)
that fonn an orthonormal set. Here i = √-1. The value of |c|2 is ______ (upto one decim al place)
The normalized wavefiinctions ψ1 and ψ2 correspond to the ground state and the first excited states of a particle in a potential. The operator A acts on the wavefiinctions as 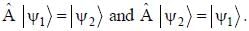 The expectation value of the operator A for the state
The expectation value of the operator A for the state 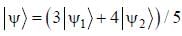 is ________. (upto two decimal places)
is ________. (upto two decimal places)
Three identical non- interacting particles, each of spin 1/2 and mass m. are moving in a one-dimensional infinite potential well given by

the energy of the lowest energy state of the system is:
A certain 2-level system lias stationary state energy 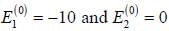 with normalized wave functions
with normalized wave functions
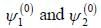 respectively. A small perturbation V is applied on the system such that V12 = 2. The wavefunction of the particle in groimd state collected upto first order, will be
respectively. A small perturbation V is applied on the system such that V12 = 2. The wavefunction of the particle in groimd state collected upto first order, will be
Given that work function of a metal is 5 eV and that photons of 9 eV are incident on it. the maximum velocity of emitted electrons is approximately______ x106 m/s (upto one decimal place)
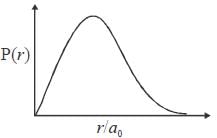
The ground state wave function of hydrogen atom is given by 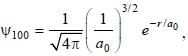 where a0 is the Bohr
where a0 is the Bohr
radius. The plot of the radial probability density P(r) for the hydrogen atom in the ground state is
A particle of mass m is constrained to move in a circular ring of radius R . When a perturbation
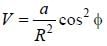
(where a is a real constant) is added, the first order collection to the ground state energy is 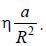 The value
The value
of η is ______ (up to one decimal place)
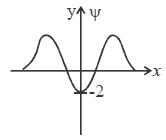
The wavefiuiction for a linear harmonic oscillator described by N 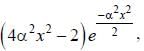 where α and N are constants, has
where α and N are constants, has
The ‘‘trial'’ wavefuction for He atom is taken as 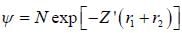 where N is the normalization factor and Z ' is the variational parameter. Expression for the energy in terms of Z ' is found to be
where N is the normalization factor and Z ' is the variational parameter. Expression for the energy in terms of Z ' is found to be
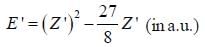
Then the lowest value of the energy ( E ') for He atom i s _________________(in a.u .)
(Round off to two decimal places)
The average distance between the electron and nucleus of a He+ (singly ionised Heliumion) in the 1s state is