Test: Ethers - JEE MCQ
20 Questions MCQ Test - Test: Ethers
Only One Option Correct Type
Direction (Q. Nos. 1-7) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Identify the final major product of the reaction sequence.
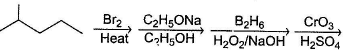
Consider the following chain of reactions :
The major product formed in the final step is
Inorganic salts are usually insoluble in organic solvents e.g, KMnO4 is insoluble in benzene. However presence of one of the following compound in benzene makes KMnO4 soluble and gives a purple coloured solution. Which is that compound?
Consider the following roadmap reaction :
The most probable structure of X is
One or More than One Options Correct Type
Direction (Q. Nos. 8 -13) This section contains 6 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct
Consider the following reaction,
Q. The expected substitution product(s) is/are
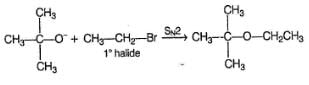
Which of the following ethers can be obtained in good yield by intermolecular dehydration of alcohols ?
If diethyl ether is left in contact of air for long time, the product formed is/are
In which of the following reaction, the major product is an acyclic ether ?
Consider the following reaction,
The correct statement(s) concerning the above transformation is/are
Consider the following reaction,
The correct statement regarding the above reaction is/are
Comprehension Type
Direction (Q. Nos. 14-16) This section contains a paragraph, describing theory, experiments, data, etc. Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
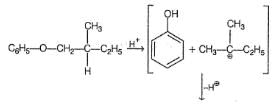
An organic compound X has molecular formula C11H16O and it can be resolved into enantiom ers. X does not evolve any gas with Na metal. X when treated with concentrated HBr gives C6H5OH and Y(C5H11Br). Y is chiral and has the same configuration as that of compound X.
Q.
Which reaction gives X as the major product?
An organic compound X has molecular formula C11H16O and it can be resolved into enantiom ers. X does not evolve any gas with Na metal. X when treated with concentrated HBr gives C6H5OH and Y(C5H11Br). Y is chiral and has the same configuration as that of compound X.
Q.
If Y is treated with C2H5ONa/C2H5OH then how many different E2 products would be formed?
An organic compound X has molecular formula C11H16O and it can be resolved into enantiom ers. X does not evolve any gas with Na metal. X when treated with concentrated HBr gives C6H5OH and Y(C5H11Br). Y is chiral and has the same configuration as that of compound X.
Q.
If X is treated with H2SO4 it undergoes a rearrangem ent to give Z as the major organic product. What is Z?
One Integer Value Correct Type
Direction (Q. Nos. 17-20) This section contains 5 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q. If a mixture of a 1-propanol and 2-butanol is heated with concentrated H2SO4 at 140°C, how many different ethers would be formed?
When 2-ethyl-3-methyl-1 -pentene is treated with CH3OH in H2SO4, how many different methoxy ethers would be formed in significant amount?
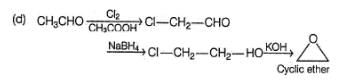
How many of the following reactions give the cyclic ether as the major organic product?