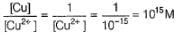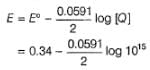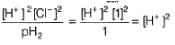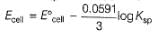Test: Applications of EMF Measurements - NEET MCQ
19 Questions MCQ Test - Test: Applications of EMF Measurements
Only One Option Correct Type
This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Q.
For, Pt(H2)/H2O, electrode potential at 298 K and 1 bar is
For the cell reaction at 298K, Cu2+(aq) + 2e- → Cu(s)
Variation of log[Cu2+ ] with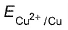 is a straight line of intercept 0.34 V on
is a straight line of intercept 0.34 V on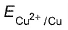 axis .Then electrode potential of the half-cell. Cu/Cu2+ (0.1 M) will be
axis .Then electrode potential of the half-cell. Cu/Cu2+ (0.1 M) will be
Given, Cu2+ + 2e- → Cu, E° = + 0.34V, Ksp of Cu(OH)2 = 1.0 x 10-19
What i s E°red of Cu2+/Cu couple at pH = 12?
EMF of the following cell is 0.67 V at 298K.
Thus, pH of the solution = 0.28 V
Given,
A3+ + e-  A2+ , E0. = 1.42 V
A2+ , E0. = 1.42 V
B4+ + 2e-  B2+ , E0. = 0.40 V
B2+ , E0. = 0.40 V
In the potentiometric titration of B2+ with A3+ the potential at the equivalence point is
For the cell of 298 K,
Zn(s) + Cu2+ (aq)  Cu(s) + Zn2+ (aq)
Cu(s) + Zn2+ (aq)
Variation of Ecell with logQ (where Q is reaction quotient ) is of the type
At what value of ratio of molar concentration of ions Ecell would be 1.1591?
Given, Ag+ + e- → Ag, 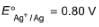
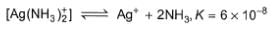
Thus, E° of the following half-cell reaction at 298 K.
Ag+ /Ag electrode is immersed in 1.00 M KCl at 298 K. Ksp (AgCl) = 1.0 x 10-10
Thus, EMF of the cell set up is
For the following cell, Pt(H2) | HCI(aq) || AgCI | Ag
Ecell = 0.2650 V at 298 K and Ecell = 0.2595 V at 308 K
Thus, heat of reaction at 298 K is
Quinhydrone electrode is an indicator electrode in contact with platinum metal and can be used in acid-base potentiometric titration
At the equivalence point, [A] = [B] and thus, EMF of the cell is dependent on pH. When NaOH is added to HCl in potentiometric titration, potentiogram is
Potential at the equivalence point in the potentiometric titration of Fe2+ with MnO4- in acidic medium is
Comprehension Type
Direction : This section contains 3 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage I
The electrochemical cell shown below is a concentration cell :
M/Mz+ (saturated solution of a sparingly soluble salt(MX2) || M2+(0.001 mol dm-3)|M
The EMF of the cell depends on the difference in concentration of M2+ ions at the two electrodes .The EMF of the cell at 298 K is 0.059 V.
Q.
Ksp mol3 dm-9 (the solubility product) of MX2 at 298 K is
(2.303 RT/F = 0.059 V at 298 K)
Passage I
The electrochemical cell shown below is a concentration cell :
M/Mz+ (saturated solution of a sparingly soluble salt(MX2) || M2+(0.001 mol dm-3)|M
The EMF of the cell depends on the difference in concentration of M2+ ions at the two electrodes .The EMF of the cell at 298 K is 0.059 V.
Q.
The value of ΔG (kJ mol-1) is (1F = 96500 C mol-1)
Passage II
The standard half-cell reduction potential of
Fe3+(aq) | Fe is -0.036V and that of OH- | Fe(OH3)(s) | Feis -0.786V.
Q.
For the determination of solubility product of Fe(OH)3 (Ksp) the appropriate cell representation and its EMF respectively are
Passage II
The standard half-cell reduction potential of
Fe3+(aq) | Fe is -0.036V and that of OH- | Fe(OH3)(s) | Feis -0.786V.
Q.
The value of loge Ksp for Fe(OH)3 at 298 K is
Passage III
Tollen's reagent is used for the detection of aldehydes.When a solution of AgNO3 is added to glucose with NH4OH, then gluconic acid is formed.
Q.
For the reaction,
Passage III
Tollen's reagent is used for the detection of aldehydes.When a solution of AgNO3 is added to glucose with NH4OH, then gluconic acid is formed.
Q.
In the presence of NH3, pH is raised to 11.Thus,
One Integer Value Correct Type
This section contains 2 questions, when worked out will result in an integer value from 0 to 9 (both inclusive)
Q.
For the following half-cell,
EMF at 298 K is 0.2364 V. What is pH?
For Cu(OH)2|Cu hall-cell at pH = 12, electrode potential is 0.0455 at 298 K.
For Cu2+ (aq) + 2e- → Cu, E° = 0.34 V. Ksp value of Cu(OH)2 is x x 10-4.
What is the value of x?



 cu2+ (aq) + 2OH- (aq)
cu2+ (aq) + 2OH- (aq)