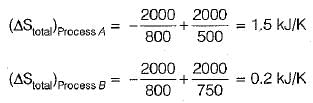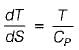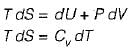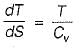Test: Entropy - 1 - Mechanical Engineering MCQ
10 Questions MCQ Test - Test: Entropy - 1
A body of constant heat capacity Cp and Initial temperature T1 is placed in contact with a heat reservoir at temperature T2 and comes to isothermal equilibrium with it. If T2 > T1 what is the entropy change of the universe?
When a system is in equilibrium, any conceivable change in entropy would be
Match List-I (Laws of thermodynamics) with List-II (Defines) the following:



Consider the processes:
Process A: A heat source at 800 K loses 2000 kJ of heat to sink at 500 K.
Process B: A heat source at 800 K loses 2000 kJ of heat to sink at 750 K.
Which of the following can be concluded?
A piston-cylinder device contains a liquid-vapor mixture of water at 300 K. During a constant- pressure process, 750 kJ of heat is transferred to the water. As a result, part of the liquid in the cylinder vaporizes. What is entropy change of the water during this process?
The slope of constant pressure line on a T-s diagram is given by


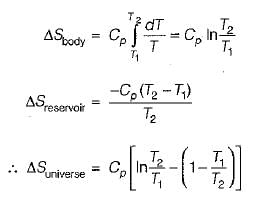
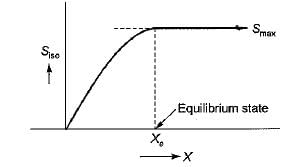
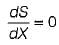 at the peak of the entropy hill.
at the peak of the entropy hill.