Important Questions (2 Marks): Acids, Bases & Salts - Class 9 MCQ
20 Questions MCQ Test - Important Questions (2 Marks): Acids, Bases & Salts
Which of the following gives the correct increasing order of acidic strength ?
Which of the following statements is true for acids?
Calcium phosphate is present in tooth enamel. Its nature is
Which of the following is(are) true when HCI (g) is passed through water ?
(i) It does not ionise in the solution as it is a covalent compound.
(ii) It ionises in the solution.
(iii) It gives both hydrogen and hydroxyl ion in the solution.
(iv) It forms hydronium ion in the solution due to the combination of hydrogen ion with water molecule.
Which one of the following can be used as an acid-base indicator by a visually impared student ?
Equal volumes of hydrochloric acid and sodium hydroxide solutions of same concentration are mixed and the pH of the resulting solution is checked with a pH paper. What would be the colour obtained ? (You may use colour guide given in figure)
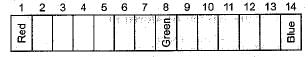
Common salt besides being used in kitchen can also be used as the raw material for making
(i) washing soda
(ii) bleaching powder
(iii) baking soda
(iv) slaked lime
Which of the following statement is not correct?
Sodium carbonate is a basic salt because it is a salt of
Which among the following is not a base ?
Which of the following statements is correct about an aqueous solution of an acid and of a base ?
(i) Higher the pH, stronger the acid
(ii) Higher the pH, weaker the acid
(iii) Lower the pH, stronger the base
(iv) Lower the pH, weaker the base
Which of the following is used for dissolution of gold ?
Which of the following salts does not contain water of crystallisation ?
In an attempt to demonstrate electrical conductivity through an electrolyte, the following apparatus was set up.
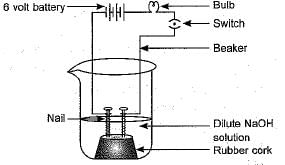
Which among the following statement(s) is (are) correct?
(i) Bulb will not glow because electrolyte is not acidic.
(ii) Bulb will glow because NaOH is a strong base: and furnishes ions for conduction.
(iii) Bulb will not glow because circuit is incomplete.
(iv) Bulb will not glow because it depends upon the type of electrolytic solution.
During the preparation of hydrogen chloride gas on a humid day, the gas is usually passed through the guard tube containing calcium chloride. The role of calcium chloride taken in the guard tube is to
Which one of the following can be used as an acid-base indicator by a visually impared (blind) Student ?
What happens when a solution of an acid is mixed with a solution of a base in a test tube ?
(i) The temperature of the solution increases.
(ii) The temperature of the solution decreases.
(iii) The temperature of the solution remains the same.
(iv) Salt formation takes place.
Which of the following phenomena will occur, when a small amount of acid is added to water?
(i) Ionisation
(ii) Neutralisation
(iii) Dilution
(iv) Salt formation
A sample of soil is mixed with water and allowed to settle. The clear supernatant solution turns the pH paper yellowish-orange. Which of the following would change the colour of this pH paper to greenish-blue?
An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change?



















