Carbon & its Compounds - 2 - UPSC MCQ
20 Questions MCQ Test - Carbon & its Compounds - 2
Which of the following represents the saponification reaction ?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which among the following are unsaturated hydrocarbons ?
(i) H3C -CH2-CH2-CH3
(ii) H3C -C = C-CH3
(iii) 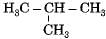
(iv) 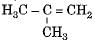
(i) H3C -CH2-CH2-CH3
(ii) H3C -C = C-CH3
(iii)
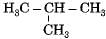
(iv)
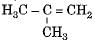
The blindness and death is caused by consuming adulterated liquor contains.
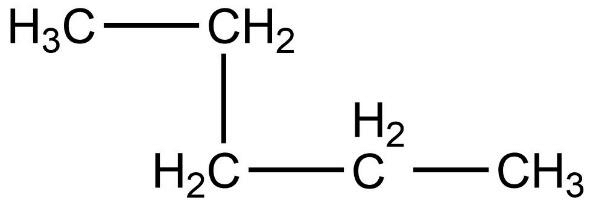
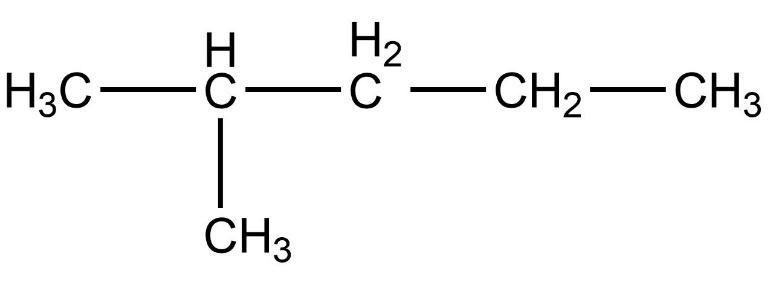
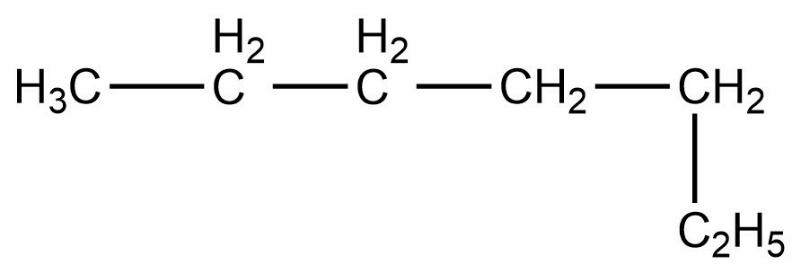
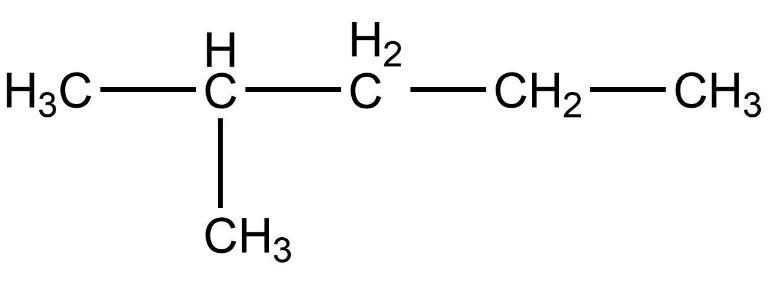
Which of the following is not a straight chain hydrocarbon ?
Which of the following does not belong to the same homologous series ?
The heteroatoms present in
CH3—CH2—O—CH2—CH2Cl are
(i) oxygen
(ii) carbon
(iii) hydrogen
(iv) chlorine
Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms, e.g., hydrogen. After the formation of four bonds, carbon attains the electronic configuration of
Ethanol reacts with sodium and forms two products. These are
Mineral acids are stronger acids than carboxylic acids because
(i) mineral acids are completely ionised.
(ii) carboxylic acids are completely ionised.
(iii) mineral acids are partially ionised.
(iv) carboxylic acids are partially ionised.
Chlorine reacts with saturated hydrocarbons at room temperature in the



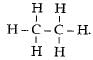 There are 7 covalent bonds in the compound.
There are 7 covalent bonds in the compound.









