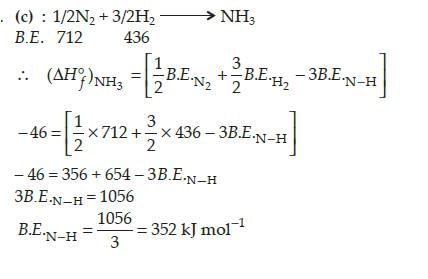Test: JEE Previous Year Questions- Thermochemistry - JEE MCQ
12 Questions MCQ Test - Test: JEE Previous Year Questions- Thermochemistry
If at 298 K the bond energies of C–H, C–C, C=C and H–H bonds are respectively 414, 347, 615 and 435 kJ mol-1, the value of enthalpy change for the reaction ;
H2C = CH2(g) + H2(g) → H3C–CH3(g) at 298 K will be – [AIEEE-2003]
The enthalpy change for a reaction does not depend upon -
[AIEEE-2003]
The enthalpies of combustion of carbon and carbon monoxide are -393.5 and -283 kJ mol-1 respectively. The enthalpy of formation of carbon monoxide per mole -
[AIEEE-2004]
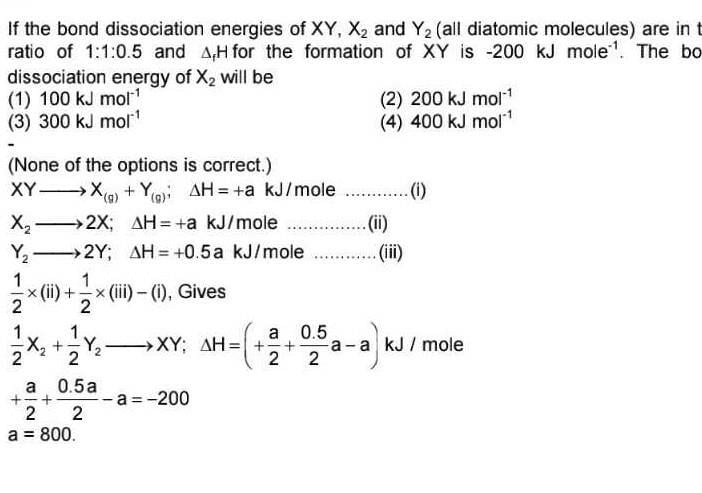
If the bond dissociation energies of XY, X2 and Y2 (all diatomic molecules) are in the ratio of 1 : 1 : 0.5 and ΔfH for the formation of XY is -200 kJ mol-1. The bond dissociation energy of X2 will be
[AIEEE-2005]
The standard enthalpy of formation (ΔfHº) at 298 K for methane, CH4(g), is -74.8 kJ mol-1. The additional information required to determine the average energy for C - H bond formation would be -
[AIEEE 2006]
Oxidising power of chlorine in aqueous solution can be determined by the parameters indicated below :
The energy involved in the conversion of Cl2(g) to Cl-(aq)
(using the data, Δdiss
kJ mol-1) will be - [AIEEE 2008]
On the basis of the following thermochemical data
The value of enthalpy of formation of OH- ion at 25ºC is -
[AIEEE 2009]
In a fuel cell methanol is used as fuel and oxygen gas is used as an oxidizer. The reaction is
At 298 K standard Gibb's energies of formation for CH3OH(l), H2O(l) and CO2(g) are -166.2, -237.2 and -394.4 kJ mol-1 respectively. If standard enthalpy of combustion of methanol is -726 kJ mol-1, efficiency of the fuel cell will be-
[AIEEE 2009]
The value of enthalpy change (DH) for the reaction C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(l)
at 27°C is -1366.5 kJ mol-1. The value of internal energy change for the above reaction at this temperature will be -
[AIEEE 2011]
Consider the reaction :
4NO2(g) + O2(g) → 2N2O5(g), ΔrH = -111kJ If N2O5(s) is formed instead of N2O5(g) in the above reaction, the ΔrH value will be : (given, ΔH of sublimation for N2O5 is 54 kJ mol-1)
[AIEEE 2011]
In view of the signs of DrG° for the following reactions :
PbO2 + Pb → 2 PbO, DrG° < 0
SnO2 + Sn → 2 SnO, DrG° > 0,
which oxidation states are more characteristic for lead and tin ?
[AIEEE 2011]
The standard enthalpy of formation of NH3 is -46.0 kJ mol-1 . If the enthalpy of formation of H2 from its atoms is -436 kJ mol-1 and that of N2 is -712 kJ mol-1 , the average bond enthalpy of N - H bond in
[AIEEE 2010]