QUIZ-1(calorimetry,thermal Expansion,thermometry)(#free Test Series) - JEE MCQ
20 Questions MCQ Test - QUIZ-1(calorimetry,thermal Expansion,thermometry)(#free Test Series)
When a copper ball is heated, the largest percentage increase will occur in its [EAMCET 1992]
A vertical column 50 cm long at 50ºC balances another column of same liquid 60 cm long at 100ºC. The coefficient of absolute expansion of the liquid is [EAMCET 1990]
The apparent coefficient of expansion of a liquid when heated in a copper vessel is C and when heated in a silver vessel is S. If A is the linear coefficient of expansion of copper, then the linear coefficient of expansion of silver is [EAMCET 1991]
When a rod is heated but prevented from expanding, the stress developed is independent of [EAMCET 1997]
Expansion during heating [CBSE PMT 1994]
On heating a liquid of coefficient of cubical expansion g in a container having coefficient of linear expansion g / 3, the level of liquid in the container will [EAMCET 1993]
540 g of ice at 0ºC is mixed with 540 g of water at 80ºC. The final temperature of the mixture is [AFMC 1994]
Water is used to cool radiators of engines, because [AFMC 2001]
How much heat energy is gained when 5 kg of water at 20ºC is brought to its boiling point (Specific heat of water = 4.2 kJ kg-1cº-1) [BHU 2001]
Heat required to convert one gram of ice at 0ºC into steam at 100?C is (given Lsteam = 536 cal/gm) [Pb. PMT 1990]
Two spheres made of same substance have diameters in the ratio 1 : 2. Their thermal capacities are in the ratio of [JIPMER 1999]
5 g of ice at 0ºC is dropped in a beaker containing 20 g of water at 40ºC. The final temperature will be [Pb. PET 2003]
The temperature of a substance increases by 27ºC. On the Kelvin scale this increase is equal to [CPMT 1993]
The resistance of a resistance thermometer has values 2.71 and 3.70 ohm at 10ºC and 100ºC. The temperature at which the resistance is 3.26 ohm is [CPMT 1994]
The temperature of the sun is measured with [Pb. PMT 1998; CPMT 1998; Pb. PET 1997, 2001]
Absolute temperature can be calculated by [AFMC 1994]
Thermoelectric thermometer is based on [CPMT 1993, 95; AFMC 1998]
A constant volume gas thermometer shows pressure reading of 50cm and 90cm of mercury at 0ºC and 100ºC respectively. When the pressure reading is 60 cm of mercury, the temperature is [MNR 1991; UPSEAT 2000; Pb. CET 2004]
If a thermometer reads freezing point of water as 20ºC and boiling point as 150ºC, how much thermometer read when the actual temperature is 60ºC [AFMC 2004]
Mercury boils at 367ºC. However, mercury thermometers are made such that they can measure temperature up to 500ºC. This is done by [CPMT 2004]




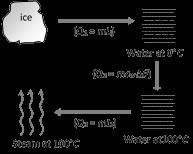 Heat required in the given process =Q1+Q2+Q3=1×80+1×1×(100−0)+1×536=716cal
Heat required in the given process =Q1+Q2+Q3=1×80+1×1×(100−0)+1×536=716cal
















