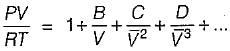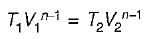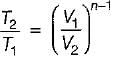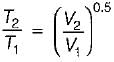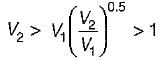Test: Properties of Gases - 3 - Mechanical Engineering MCQ
15 Questions MCQ Test - Test: Properties of Gases - 3
The characteristic equation of gases PV = mRT holds good for
A gas which obeys kinetic theory perfectly is known as
Variation of pressure and volume at constant temperature are correlated through
The work given out during expansion process in a closed system will increase when the value of n (the index of compression)
The work required for compression in a closed system increases the value of n (the index of compression)
In thermodynamics analysis, a pure substance is that which
Which one of the following PV - T diagram correctly represents that of an ideal gas?
If 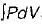 and
and 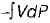 for a thermodynamic system of an ideal gas on evaluation give the same quantify during a process, then the process under gone by the system is
for a thermodynamic system of an ideal gas on evaluation give the same quantify during a process, then the process under gone by the system is
A vessel of volume 1m3 contains oxygen (molecular weight = 32) at P = 1 bar and T = 47°C. The mass of oxygen in the vessel is (take universal gas constant as 8314 J/mol-K)
Nitrogen at an initial state of 10 bar, 1 m3, and 300 K is expanded isothermally to a final volume of 2m3. The p-v-T relation is 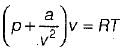 , where a > 0. The final pressure.
, where a > 0. The final pressure.
Which combination of the following statements is correct?
P : A gas cools upon expansion only when its Joule-Thomson coefficient is positive in the temperature range of expansion.
Q : For a system undergoing a process, its entropy remains constant only when the process is reversible.
R : The work done by a closed system in an adiabatic is a point function S : A liquid expands upon freezing when the slope of its fusion curve on pressure-temperature diagram is negative.
Van der Waal’s equation of state is given by
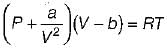
The value of R in terms of Pc, Vc & Tc is
An ideal gas undergoes expansion according to the process PV0.5 = constant. The temperature of'the gas during expansion process



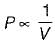
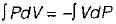
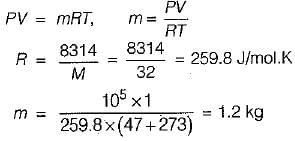
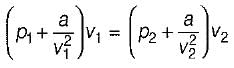
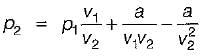
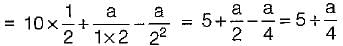
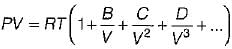 is known as
is known as