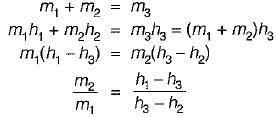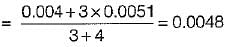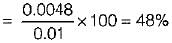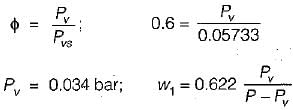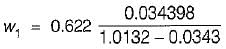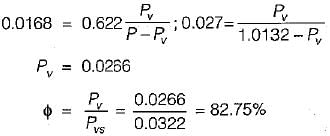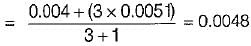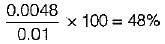Test: Properties of Moist Air - 3 - Mechanical Engineering MCQ
10 Questions MCQ Test - Test: Properties of Moist Air - 3
If the measured wet-bulb temperature and the thermodynamic wet-bulb temperature are equal, then the non-dimensional number with a value of unity is the
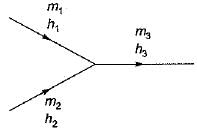
If two streams of air containing m1 and m2 mass of dry air and enthalpy h1 and h2 are mixed adiabaticaily then
The barometer pressure is 1.03 bar, the partial pressure of air is 1 bar and the saturation partial pressure of water vapour at the same dry bulb temperature is 0.05 bar. What is the relative humidity?
Air at state 1 (DPT = 10°C, w = 0.0040 kg/kg) mixes with air at state 2 (DPT = 18°C, w = 0.0051 kg/kg) in the ratio 1 to 3 by weight. The degree of saturation (%) of the mixture is (The specific humidity of saturated air at 13.6°C. w = 0.01 kg/kg)
For a typical sample of ambient air (at 30°C, 75% relative humidity and standard atmospheric pressure), the amount of moisture in kg per kg of dry air will be approximately
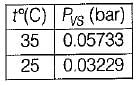
5 gm of water vapour per kg of air is removed and temperature of air after removing water vapour is 25°C DBT.
Find the relative humidity. Use following table,
Air at state 1 (DPT = 1°C, w= 0.0040 kg/kg of da) mixes with air at state 2 (DPT = 18°C, w = 0.0051 kg/kg of da) in the ratio of 1 to 3 by weight the degree of saturation (%) of the mixture is (The specific humidity of saturated air at 13.6°C, w = 0.01 kg/kg of da)
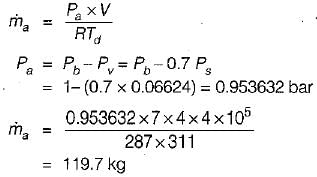
A room 7m x 4m x 4m is occupied by an air- water vapour mixture at 38°C. The atmospheric pressure is 1 bar & relative humidity is 70%. The mass of air is (take Ps at 38°C = 0.06624 bar)