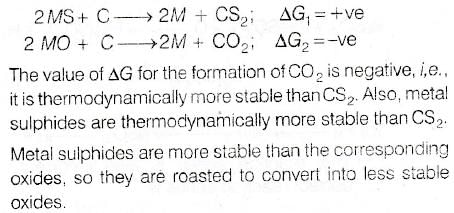Test: JEE Previous Year Questions- General Principles & Processes of Isolation of Elements - JEE MCQ
8 Questions MCQ Test - Test: JEE Previous Year Questions- General Principles & Processes of Isolation of Elements
The metal extracted by leaching with cyanide is :
[AIEEE-2002]
When the sample of Cu with Zn impurity is to be purfied by electrolysis, the appropriate electrodes are :
Cathode Anode
[AIEEE-2002]
Aluminium is industrially prepared by -
[AIEEE-2002]
The substance not likely to contain CaCO3 is –
[AIEEE-2003]
Which one of the following ores is best concentrated by froath-flotation method ?
[AIEEE-2004]
During the process of electrolytic refining of copper, some metals present as impurity settle as 'anode mud'. These are –
[AIEEE-2005]
Heating mixture of Cu2O and Cu2S will give –
[AIEEE-2005]
Which of the following factors is of no significance for roasting sulphide ores to the oxides and not subjecting the sulphide ores to carbon reduction directly ?
[AIEEE-2008]