Test: JEE Previous Year Questions- p-Block Elements - JEE MCQ
9 Questions MCQ Test - Test: JEE Previous Year Questions- p-Block Elements
The correct no. of lone pairs on the central atom of compounds XeF2, XeF4 & XeF6are respectively-
[AIEEE-2002]
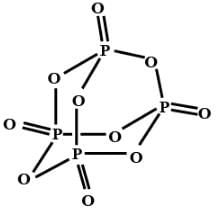
The no. of s bonds in the compound P4O10 is -
[AIEEE-2002]
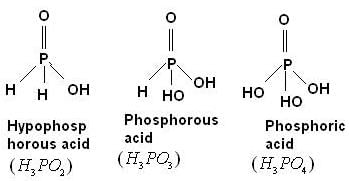
The number of hydrogen atoms (s) attached to phosphorus atom in hypophosphorous acid is –
[AIEEE-2005]
What products are expected from the disproportionation reaction of hypochlorous acid –
[AIEEE- 2006]
Which one of the following statements regarding helium is incorrect
[AIEEE 2004]
Which one of the following reactions of Xenon compounds is not feasible ?
[AIEEE-2009]
In which of the following arrangements, the sequence is not strictly according to the property written against it ?
[AIEEE-2009]
Identify the incorrect statement from the following -
[AIEEE-2011]
The number of types of bonds between two carbon atoms in calcium carbide is -
[AIEEE-2011]