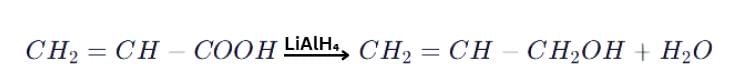Test: JEE Previous Year Questions- Alcohols - JEE MCQ
10 Questions MCQ Test - Test: JEE Previous Year Questions- Alcohols
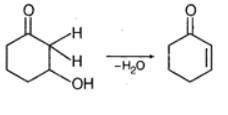
Which of the following compounds undergoes maximum dehydration?
When CH2=CHCOOH is reduced with LiAlH4, the compound obtained will be
Among the following compounds which can be dehydrated very easily?
For which of the following parameters the structural isomers C2H5OH and CH3OCH3 would be expected to have the same values ? (Assume ideal behaviour)
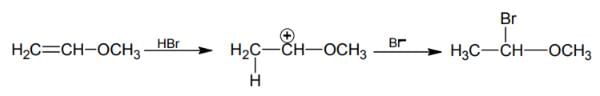
HBr reacts with CH2 = CH – OCH3 under anhydrous conditions at room temperature to give
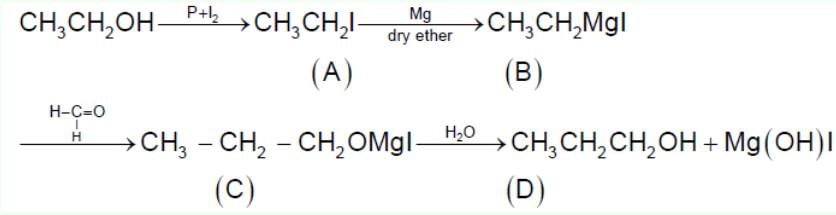
In the following sequence of reactions, 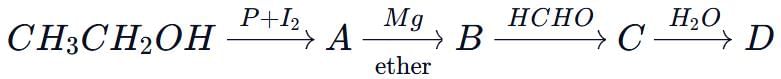 , what is compound D?
, what is compound D?
A liquid was mixed with ethanol and a drop of concentrated H2SO4 was added. A compound with a fruity smell was formed. The liquid was
From amongst the following alcohols the one that would react fastest with conc HCl and anhydrous ZnCl2 is
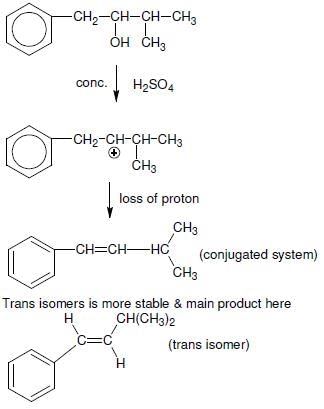
The main product of the following reaction is
Consider the following reaction :
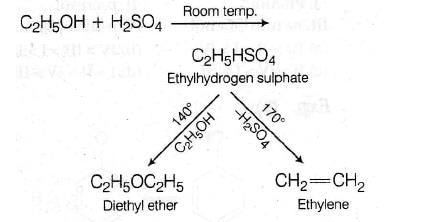
C2H5OH + H2SO4 → Product
Among the following which one cannot be formed as a product under any conditions ?