Test: JEE Previous Year Questions- Carbonyl Group - JEE MCQ
18 Questions MCQ Test - Test: JEE Previous Year Questions- Carbonyl Group
Q.1 End product of the following reaction is – [AIEEE-2002]
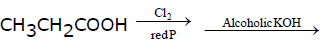
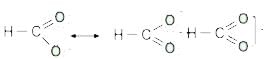
In the anion HCOO- the two carbon-oxygen bonds are found to be of equal length. What is the reason for it ? [AIEEE-2003]
When CH2 = CHCOOH is reduced with LiAlH4, the compound obtained is: [AIEEE-2003]
The general formula CnH2nO2 could be for open chain : [AIEEE-2003]
Which one of the following does not have sp2 hybridized carbon ? [AIEEE-2004]
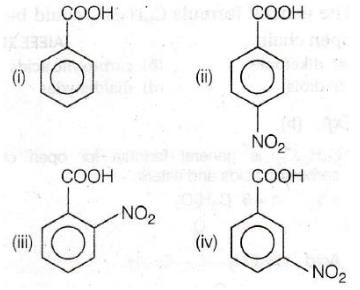
Consider the acidity of the carboxylic acids : [AIEEE-2004]
(a) PhCOOH
(b) o – NO2C6H4COOH
(c) p – NO2C6H4COOH
(d) m – NO2C6H4COO
Which of the following order is correct ?
Rate of the reaction :
is fastest when Z is : [AIEEE-2004]
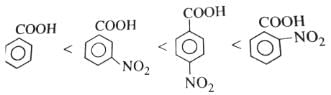
Consider the acidity of the carboxylic acids :
(1) PhCOOH
(2) 0-NO2C6H4COOH
(3) p-NO2C6H4COOH
(4) m-NO2C6H4COOH
Which of the following order is correct ? [AIEEE-2004]
On mixing ethyl acetate with aqueous sodium chloride, the composition of the resultant solution is :
[AIEEE-2004]
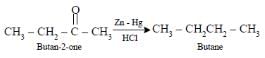
Which one of the following is reduced with zinc and hydrochloric acid to give the corresponding
hydrocarbon : [AIEEE-2004]
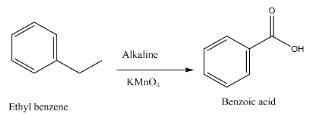
The compound formed as a result of oxidation of ethyl benzene by KMnO4 is – [AIEEE-2007]
A liquid was mixed with ethanol and a drop of concentrated H2SO4 was added. A compound with a fruity
smell was formed. The liquid was : [AIEEE-2009]
Reaction
Primary amine + CHCl3 + KOH → product, here product will be - [AIEEE-2002]
The compound formed in the positive test for nitrogen with the Lassaigne solution of an organic compound is - [AIEEE-2004]
Which one of the following methods is neither meant for the synthesis nor for separation of amines ?
[AIEEE-2005]
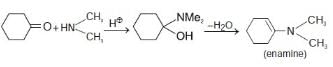
Reaction of cyclohexanone with dimethylamine in the presence of catalytic amount of an acid forms a
compound if water during the reaction is continuously removed. The compound formed is generally known
as – [AIEEE-2005]
In the chemical reaction, CH3CH2NH2 + CHCl3 + 3KOH → (A) + (B) + 3H2O, the compounds (A) and (B)
are respectively - [AIEEE-2007]