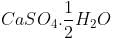NTSE Test: Acids, Bases & Salts - Class 9 MCQ
15 Questions MCQ Test - NTSE Test: Acids, Bases & Salts
The compound which does not exist as hydrate form:
Carbon dioxide reacts with calcium hydroxide to form calcium carbonate and water. Ca(OH)2 + CO2 → CaCO3 + H2O This reaction is known as
Assertion: Metal oxides react with acid to form salt and water.
Reason: Most of the metal oxides are acidic in nature.
When sodium hydroxide reacts with aluminium metal:
A salt associated with the water of crystallization is known as a:
To protect tooth decay, we are advised to brush our teeth regularly. The nature of the toothpaste commonly used is:
The pH of a compound is found to be 9. The compound can be:
Which impurity (as a salt) is associated with table salt obtained from seawater?
Which among the following represents the chemical formula for ‘Plaster of Paris’?