Test: Carbon and its compounds (Medium) - Class 10 MCQ
20 Questions MCQ Test - Test: Carbon and its compounds (Medium)
How would you name the following compound?CH3-CH2-Br
Trivial name of 2, 3-dihydroxy butanedioic acid is-
Washing soaps produce scum with hard water and not much foam because hard water contains:
Chlorine Reacts With Saturated Hydrocarbons At Room Temperature In The:
Oils On Treating With Hydrogen In The Presence Of Palladium Or Nickel Catalyst Form Fats. This Is An Example Of:
CH3 – CH2 – OH + Alkaline KMnO4 + Heat → CH3 – COOH. In The Above Given Reaction, Alkaline KMnO4 Acts As
Read The Following Sentences Carefully, And Choose The Incorrect One:
Pooja Is Writing Some Statements But She Confused To Know Whether The Statements Are Correct Or Not? If You Know The Answer To This Question, Then Tell Her:
How many double bonds are there in a saturated hydrocarbon?
Hydrocarbons are mainly composed of which of these?
Which of the following statements are usually correct for carbon compounds? These
(i) are good conductors of electricity
(ii) are poor conductors of electricity
(iii) have strong forces of attraction between their molecules
(iv) do not have strong forces of attraction between their molecules
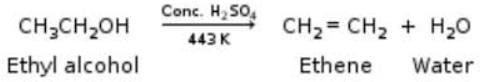
When ethanol is heated with con, sulphuric acid at 170°C, it gets converted into ethene. In this reaction concentrated sulphuric acid acts as :
Why does carbon form compounds mainly by covalent bonding?
What is the total percentage of carbon present in the human body?
In the atmosphere, carbon exists in the form of _________.
Correct percentage of CO2 present in the atmosphere is