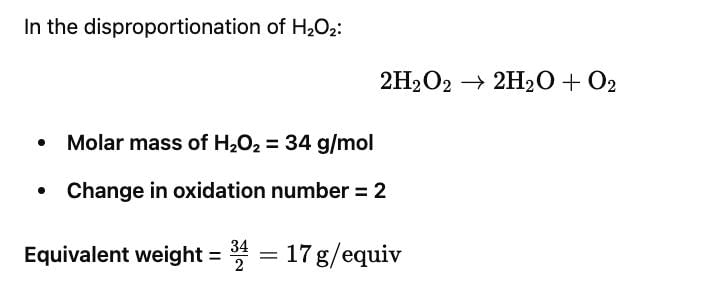Test: Hydrogen Peroxide (Old NCERT) - JEE MCQ
15 Questions MCQ Test - Test: Hydrogen Peroxide (Old NCERT)
What is the chemical formula of hydrogen peroxide?
What is molarity in terms of volume strength of hydrogen peroxide?
In which of the following, is hydrogen peroxide not stored?
Hydrogen peroxide is ______________ in nature.
What does hydrogen peroxide liberate from potassium iodide?
90% of hydrogen peroxide is used as fuel in ______________
Which of the following is not a use of hydrogen peroxide?
What is the acute angle between oxygen and hydrogen in the solid form of hydrogen peroxide?
Normality of hydrogen peroxide is given by 2 and what is its volume strength?
Which of the following is the relation between percent strength and volume strength of hydrogen peroxide?
What is a solution of hydrogen peroxide generally labeled as?
In the disproportionation Hydrogen peroxide, forming H2O and O2 its equivalent weight is