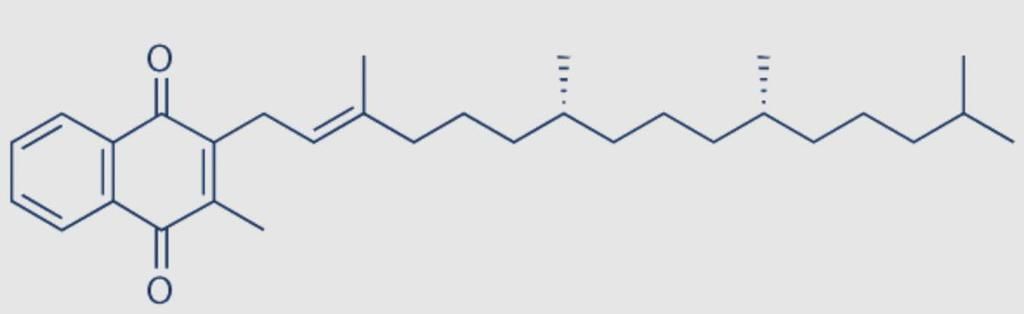
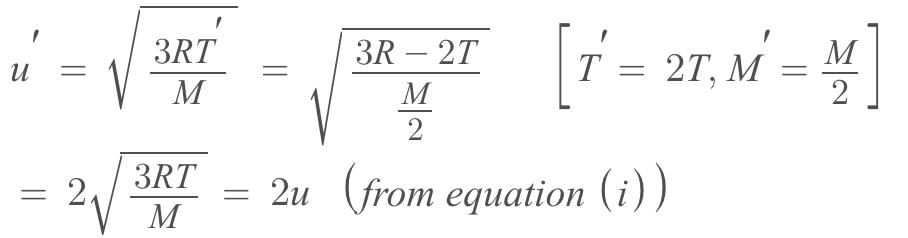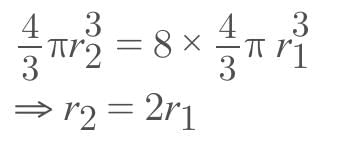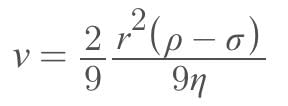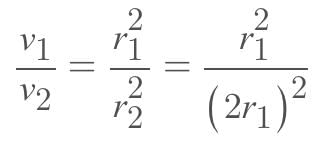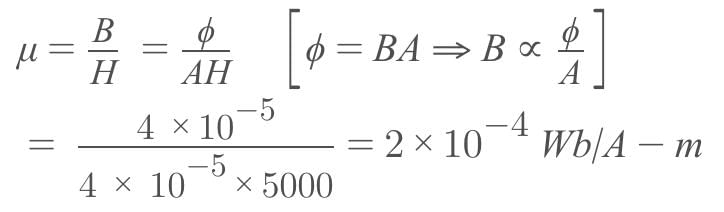UGEE SUPR Mock Test- 6 - JEE MCQ
30 Questions MCQ Test - UGEE SUPR Mock Test- 6
A solid sphere rolls down from the top of an inclined plane, 7m high, without slipping. Its Linear speed at the foot of plane is g = 10 m/s2)
Which of the following is the dimensional formula for electric polarisation?
A vector P has X and Y components of magnitude 2 units and 4 units respectively. A vector Q along the negative X-axis has magnitude 6 units. The vector (Q - P) will be
When a large bubble rises from the bottom of a water lake to its surface, then its radius doubles, if the atmospheric pressure is equal to the pressure of height Hof a certain water column, then the depth of the lake will be
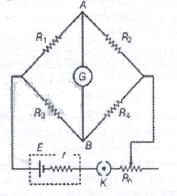
In the network shown cell E has internal resistance r and the galvanometer shows zero deflection. If the cell is replaced by a new cell of 2E and internal resistance 3r keeping ling everything else identical, then
If the radius of the circular path and frequency of revolution of a particle of mass m are doubled, then the change in its kinetic energy will be (Ei and Er are the Initial and final kinetic energies of the particle respectively.)
The rms speed of oxygen molecule in a gas is u, If the temperature is doubted and the molecules dissociates into two atoms, the rms speed will be
Eight identical drops of water faking through the air with a uniform velocity of 10 cm/s combines to form a single drop of big size, then the terminal velocity of the big drop will be
A body of mass m is performing a UCM in a circle of radius r with speed v. The work done by the centripetal force in moving it through (2/3)rd of the circular path is
A magnetizing field of 5000 A/m produces a magnetic flux of 4 x 10-5 Wb in an iron rod of cross-sectional area 0.4 cm2. The permeability of the rod in Wb/A-m, is
A block of mass M is pulled along a smooth horizontal surface with a rope of mass m by force F. The acceleration of the block will be
In hydrogen emission spectrum , for any series, the principthe al quantum number is n.
Corresponding maximum wavelength λ is (R = Rydbergs constant)
The total energy of a simple harmonic oscillator is proportional to
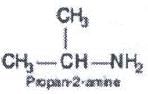
How many primary amines are possible for the molecular formula C3H9N?
A polymer which becomes soft on heating and hard on cooling, belongs to class of
Which bond in a molecule of ethyl magnesium bromide is ionic in nature?
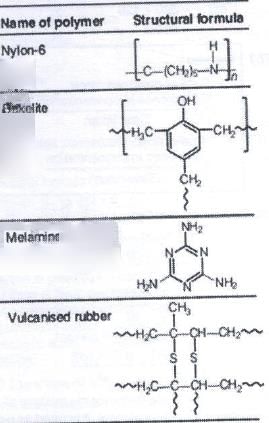
Which among the following polymer does not show cross linking in it?
The conversion of 2-methylpropan-1-ol to 2-methylpropan-2-ol is
The effective atomic number of Iron (Z = 26) in [Fe{CN)6)-3 is
Based on first Jaw of thermodynamics which of the following is correct


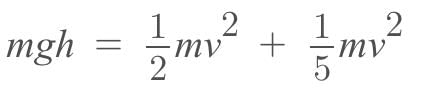

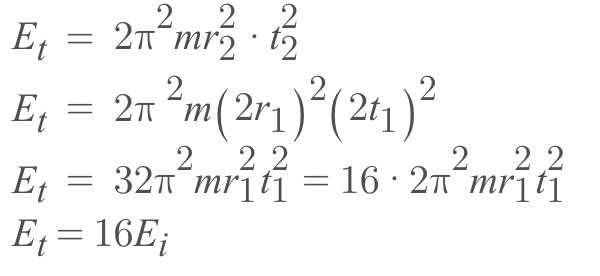
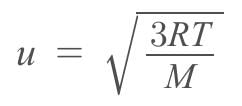 …(i)
…(i)