Case Based Questions Test: Biomolecules - 2 - NEET MCQ
10 Questions MCQ Test - Case Based Questions Test: Biomolecules - 2
Read the passage given below and answer the following questions:
When a protein in its native form, is subjected to physical changes like change in temperature or chemical changes like change in pH, the hydrogen bonds are disturbed. Due to this, globules unfold and helix get uncoiled and protein loses its biological activity. This is called denaturation of protein.
The denaturation causes change in secondary and tertiary structures but primary structures remains intact.
Examples of denaturation of protein are coagulation of egg white on boiling, curding of milk, formation of cheese when an acid is added to milk.
Q. α-helix and β-pleated structures of proteins are classified as
When a protein in its native form, is subjected to physical changes like change in temperature or chemical changes like change in pH, the hydrogen bonds are disturbed. Due to this, globules unfold and helix get uncoiled and protein loses its biological activity. This is called denaturation of protein.
The denaturation causes change in secondary and tertiary structures but primary structures remains intact.
Examples of denaturation of protein are coagulation of egg white on boiling, curding of milk, formation of cheese when an acid is added to milk.
Q. α-helix and β-pleated structures of proteins are classified as
Read the passage given below and answer the following questions:
When a protein in its native form, is subjected to physical changes like change in temperature or chemical changes like change in pH, the hydrogen bonds are disturbed. Due to this, globules unfold and helix get uncoiled and protein loses its biological activity. This is called denaturation of protein.
The denaturation causes change in secondary and tertiary structures but primary structures remains intact.
Examples of denaturation of protein are coagulation of egg white on boiling, curding of milk, formation of cheese when an acid is added to milk.
Q. Cheese is a
When a protein in its native form, is subjected to physical changes like change in temperature or chemical changes like change in pH, the hydrogen bonds are disturbed. Due to this, globules unfold and helix get uncoiled and protein loses its biological activity. This is called denaturation of protein.
The denaturation causes change in secondary and tertiary structures but primary structures remains intact.
Examples of denaturation of protein are coagulation of egg white on boiling, curding of milk, formation of cheese when an acid is added to milk.
Q. Cheese is a
Read the passage given below and answer the following questions:
EVIDENCE FOR THE FIBROUS NATURE OF DNA
The basic chemical formula of DNA is now well established. As shown in Figure 1 it consists of a very long chain, the backbone of which is made up of alternate sugar and phosphate groups, joined together in regular 3’ 5’ phosphate di-ester linkages. To each sugar is attached a nitrogenous base, only four different kinds of which are commonly found in DNA. Two of these---adenine and guanine--- are purines, and the other two thymine and cytosine-are pyrimidines. A fifth base, 5-methyl cytosine, occurs in smaller amounts in certain organisms, and a sixth, 5-hydroxy-methyl-cytosine, is found instead of cytosine in the T even phages. It should be noted that the chain is unbranched, a consequence of the regular internucleotide linkage. On the other hand the sequence of the different nucleotides is, as far as can be ascertained, completely irregular. Thus, DNA has some features which are regular, and some which are irregular. A similar conception of the DNA molecule as a long thin fiber is obtained from physicochemical analysis involving sedimentation, diffusion, light scattering, and viscosity measurements. These techniques indicate that DNA is a very asymmetrical structure approximately 20 A wide and many thousands of angstroms long. Estimates of its molecular weight currently center between 5 X106 and X107 (approximately 3 X104 nucleotides). Surprisingly each of these measurements tend to suggest that the DNA is relatively rigid, a puzzling finding in view of the large number of single bonds (5 per nucleotide) in the phosphate-sugar back bone. Recently these indirect inferences have been confirmed by electron microscopy.
Q. Purines present in DNA are:
EVIDENCE FOR THE FIBROUS NATURE OF DNA
The basic chemical formula of DNA is now well established. As shown in Figure 1 it consists of a very long chain, the backbone of which is made up of alternate sugar and phosphate groups, joined together in regular 3’ 5’ phosphate di-ester linkages. To each sugar is attached a nitrogenous base, only four different kinds of which are commonly found in DNA. Two of these---adenine and guanine--- are purines, and the other two thymine and cytosine-are pyrimidines. A fifth base, 5-methyl cytosine, occurs in smaller amounts in certain organisms, and a sixth, 5-hydroxy-methyl-cytosine, is found instead of cytosine in the T even phages. It should be noted that the chain is unbranched, a consequence of the regular internucleotide linkage. On the other hand the sequence of the different nucleotides is, as far as can be ascertained, completely irregular. Thus, DNA has some features which are regular, and some which are irregular. A similar conception of the DNA molecule as a long thin fiber is obtained from physicochemical analysis involving sedimentation, diffusion, light scattering, and viscosity measurements. These techniques indicate that DNA is a very asymmetrical structure approximately 20 A wide and many thousands of angstroms long. Estimates of its molecular weight currently center between 5 X106 and X107 (approximately 3 X104 nucleotides). Surprisingly each of these measurements tend to suggest that the DNA is relatively rigid, a puzzling finding in view of the large number of single bonds (5 per nucleotide) in the phosphate-sugar back bone. Recently these indirect inferences have been confirmed by electron microscopy.
Q. Purines present in DNA are:
Read the passage given below and answer the following questions:
EVIDENCE FOR THE FIBROUS NATURE OF DNA
The basic chemical formula of DNA is now well established. As shown in Figure 1 it consists of a very long chain, the backbone of which is made up of alternate sugar and phosphate groups, joined together in regular 3’ 5’ phosphate di-ester linkages. To each sugar is attached a nitrogenous base, only four different kinds of which are commonly found in DNA. Two of these---adenine and guanine--- are purines, and the other two thymine and cytosine-are pyrimidines. A fifth base, 5-methyl cytosine, occurs in smaller amounts in certain organisms, and a sixth, 5-hydroxy-methyl-cytosine, is found instead of cytosine in the T even phages. It should be noted that the chain is unbranched, a consequence of the regular internucleotide linkage. On the other hand the sequence of the different nucleotides is, as far as can be ascertained, completely irregular. Thus, DNA has some features which are regular, and some which are irregular. A similar conception of the DNA molecule as a long thin fiber is obtained from physicochemical analysis involving sedimentation, diffusion, light scattering, and viscosity measurements. These techniques indicate that DNA is a very asymmetrical structure approximately 20 A wide and many thousands of angstroms long. Estimates of its molecular weight currently center between 5 X106 and X107 (approximately 3 X104 nucleotides). Surprisingly each of these measurements tend to suggest that the DNA is relatively rigid, a puzzling finding in view of the large number of single bonds (5 per nucleotide) in the phosphate-sugar back bone. Recently these indirect inferences have been confirmed by electron microscopy.
Q. DNA has a ___________ backbone
Read the passage given below and answer the following questions:
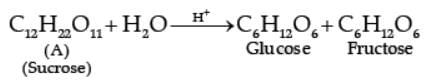
The two monosaccharides are joined together by an oxide linkage formed by the loss of a water molecule. Such a linkage between two monosaccharide units through oxygen atom is called glycosidic linkage. In disaccharides, if the reducing groups of monosaccharides i.e., aldehydic or ketonic groups are bonded, these are non-reducing sugars, e.g., sucrose. On the other hand, sugars in which these functional groups are free, are called reducing sugars, for example, maltose and lactose.
A non reducing disaccharide ‘A’ on hydrolysis with dilute acid gives an equimolar mixture of D–(+)– glucose and D-(-)-Fructose.
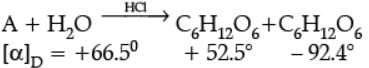
Q. In the above reaction, reactant ‘A’ is:
Read the passage given below and answer the following questions:
The two monosaccharides are joined together by an oxide linkage formed by the loss of a water molecule. Such a linkage between two monosaccharide units through oxygen atom is called glycosidic linkage. In disaccharides, if the reducing groups of monosaccharides i.e., aldehydic or ketonic groups are bonded, these are non-reducing sugars, e.g., sucrose. On the other hand, sugars in which these functional groups are free, are called reducing sugars, for example, maltose and lactose.
A non reducing disaccharide ‘A’ on hydrolysis with dilute acid gives an equimolar mixture of D–(+)– glucose and D-(-)-Fructose.
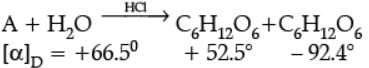
Q. Name the linkage that holds the two units in the disaccharide ?
Read the passage given below and answer the following questions:
The sequence of bases in m-RNA are read in a serial order in groups of three at a time. Each triplet of nucleotides (having a specific sequence of bases) is known as codon. Each codon specifies one amino acid. Many amino acids have more than one codons. The amino acids are brought to the mRNA by another type of RNA and called tRNA. Each amino acid has atleast one corresponding tRNA. At one end of the tRNA molecule is a trinucleotide base sequence that is complementary to some trinucleotide base sequence on mRNA.
Q. Which of the following nitrogen bases is not present in RNA?
Read the passage given below and answer the following questions:
The sequence of bases in m-RNA are read in a serial order in groups of three at a time. Each triplet of nucleotides (having a specific sequence of bases) is known as codon. Each codon specifies one amino acid. Many amino acids have more than one codons. The amino acids are brought to the mRNA by another type of RNA and called tRNA. Each amino acid has atleast one corresponding tRNA. At one end of the tRNA molecule is a trinucleotide base sequence that is complementary to some trinucleotide base sequence on mRNA.
Q. Each codon specifies:
Read the passage given below and answer the following questions:
Adenosine triphosphate (ATP) is the energy carrying molecule found in the cells of all living things. ATP captures chemical energy obtained from the breakdown of food molecules and releases it to fuel other cellular processes. ATP is a nucleotide that consists of three main structures: the nitrogenous base, adenine; the sugar, ribose; and a chain of three phosphate groups bound to ribose. The phosphate tail of ATP is the actual power source which the cell taps. Available energy is contained in the bonds between the phosphates and is released when they are broken, which occurs through the addition of a water molecule (a process called hydrolysis). Usually only the outer phosphate is removed from ATP to yield energy; when this occurs ATP is converted to adenosine diphosphate (ADP), the form of the nucleotide having only two phosphates.
The importance of ATP (adenosine triphosphate) as the main source of chemical energy in living matter and its involvement in cellular processes has long been recognized. The primary mechanism whereby higher organisms, including humans, generate ATP is through mitochondrial oxidative phosphorylation. For the majority of organs, the main metabolic fuel is glucose, which in the presence of oxygen undergoes complete combustion to CO2 and H2O: C6H12O6 + 6O2 → 6O2 + 6H2O + energy
The free energy (ΔG) liberated in this exergonic (ΔG is negative) reaction is partially trapped as ATP in two consecutive processes: glycolysis (cytosol) and oxidative phosphorylation (mitochondria). The first produces 2 mol of ATP per mol of glucose, and the second 36 mol of ATP per mol of glucose. Thus, oxidative phosphorylation yields 17-18 times as much useful energy in the form of ATP as can be obtained from the same amount of glucose by glycolysis alone.
The efficiency of glucose metabolism is the ratio of amount of energy produced when 1 mol of glucose oxidised in cell to the enthalpy of combustion of glucose. The energy lost in the process is in the form of heat. This heat is responsible for keeping us warm.
Q. Cellular oxidation of glucose is a:
Read the passage given below and answer the following questions:
Adenosine triphosphate (ATP) is the energy carrying molecule found in the cells of all living things. ATP captures chemical energy obtained from the breakdown of food molecules and releases it to fuel other cellular processes. ATP is a nucleotide that consists of three main structures: the nitrogenous base, adenine; the sugar, ribose; and a chain of three phosphate groups bound to ribose. The phosphate tail of ATP is the actual power source which the cell taps. Available energy is contained in the bonds between the phosphates and is released when they are broken, which occurs through the addition of a water molecule (a process called hydrolysis). Usually only the outer phosphate is removed from ATP to yield energy; when this occurs ATP is converted to adenosine diphosphate (ADP), the form of the nucleotide having only two phosphates.
The importance of ATP (adenosine triphosphate) as the main source of chemical energy in living matter and its involvement in cellular processes has long been recognized. The primary mechanism whereby higher organisms, including humans, generate ATP is through mitochondrial oxidative phosphorylation. For the majority of organs, the main metabolic fuel is glucose, which in the presence of oxygen undergoes complete combustion to CO2 and H2O: C6H12O6 + 6O2 → 6O2 + 6H2O + energy
The free energy (ΔG) liberated in this exergonic (ΔG is negative) reaction is partially trapped as ATP in two consecutive processes: glycolysis (cytosol) and oxidative phosphorylation (mitochondria). The first produces 2 mol of ATP per mol of glucose, and the second 36 mol of ATP per mol of glucose. Thus, oxidative phosphorylation yields 17-18 times as much useful energy in the form of ATP as can be obtained from the same amount of glucose by glycolysis alone.
The efficiency of glucose metabolism is the ratio of amount of energy produced when 1 mol of glucose oxidised in cell to the enthalpy of combustion of glucose. The energy lost in the process is in the form of heat. This heat is responsible for keeping us warm.
Q. Which of the following statements is true?