Assertion & Reason Test: Coordination Compounds - NEET MCQ
10 Questions MCQ Test - Assertion & Reason Test: Coordination Compounds
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): Toxic metal ions are removed by the chelating ligands.
Reason (R): Chelate complexes tend to be more stable.
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
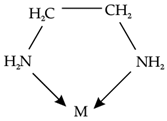
Assertion: A mong [CO(NH3)6]3+ and [CO(en)3]3+, coordination compound [CO(en)3]3+ is a more stable complex.
Reason: Because (en) is a chelating ligand/bidentate ligand.
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): [Cr(H2O)6]Cl2 and [Fe(H2O)6]Cl2 are reducing in nature.
Reason (R): Unpaired electrons are present in their d-orbitals.
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): Low spin tetrahedral complexes are rarely observed.
Reason (R): Crystal field splitting is less than pairing energy for tetrahedral complexes.
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): Linkage isomerism arises in coordination compounds containing ambidentate ligand.
Reason (R): Ambidentate ligand has two different donor atoms.
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): [Fe(CN)6]3− ion shows magnetic moment corresponding to two unpaired electrons.
Reason (R): Because it has d2sp3 type hybridisation.
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism.
Reason (R): Geometrical isomerism is not shown by complexes of coordination number 6.
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion : NF3 is a weaker ligand than N(CH3)3.
Reason : NF3 ionizes to give F– ions in aqueous solution.
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion : [Ti(H2O)6]3+ is coloured while [Sc(H2O)6]3+ is colourless.
Reason : d-d transition is not possible in [Sc(H2O)6]3+.
Directions: These questions consist of two statements, each printed as Assertion and Reason. While answering these questions, you are required to choose any one of the following four responses.
Assertion : [Fe(CN)6]3– is weakly paramagnetic while [Fe(CN)6]4– is diamagnetic.
Reason : [Fe(CN)6]3– has +3 oxidation state while [Fe(CN)6]4– has +2 oxidation state.