Case Based Questions Test: The s-Block Elements (Old NCERT) - NEET MCQ
8 Questions MCQ Test - Case Based Questions Test: The s-Block Elements (Old NCERT)
Lithium and Beryllium differ from other elements of their groups. The solubility of the ionic compounds depends upon lattice enthalpy and hydration energy. Smaller cations have greater hydration enthalpy. The salts of alkaline earth metals are extensively hydrated. Now, answer the given questions (i) to (iv):
Q. The order of solubility of lithium halide in polar solvents is:
Lithium and Beryllium differ from other elements of their groups. The solubility of the ionic compounds depends upon lattice enthalpy and hydration energy. Smaller cations have greater hydration enthalpy. The salts of alkaline earth metals are extensively hydrated. Now, answer the given questions (i) to (iv):
Q. The incorrect trend in alkali metal is
Lithium and Beryllium differ from other elements of their groups. The solubility of the ionic compounds depends upon lattice enthalpy and hydration energy. Smaller cations have greater hydration enthalpy. The salts of alkaline earth metals are extensively hydrated. Now, answer the given questions (i) to (iv):
Q. The hydroxide which has maximum solubility in water?
Lithium and Beryllium differ from other elements of their groups. The solubility of the ionic compounds depends upon lattice enthalpy and hydration energy. Smaller cations have greater hydration enthalpy. The salts of alkaline earth metals are extensively hydrated. Now, answer the given questions (i) to (iv):
Q. Which of the following forms only the normal oxide M2O on heating in air?
The lighter alkaline earth metals are very important in animal and plant physiology. Magnesium functions as the active center in some enzymes and calcium salts take a structural role in bones. The alkaline earth metals have the second lowest first ionisation energies in their respective periods. The ions of these elements perform important biological functions like maintenance of ion balance and nerve impulse conduction. The given questions (i) to (iv) consist of an assertion (A) and reason (R). Choose the correct option.
Assertion: The second ionisation enthalpies of the alkaline earth metals are smaller than the metals.
Reason: The second electron is to be removed from the cation have inert gas configuration.
Read the passage given below and answer the following questions.
Alkali metals have the lowest ionization energy in their corresponding period in periodic table because they have large size which results in a large distance between the nucleus and the outermost electron. Ionization energy of alkali metals decreases from Li to Cs due to increase in atomic size. First ionization energy of alkali metals is very low, but they have very high value of second ionization energy.
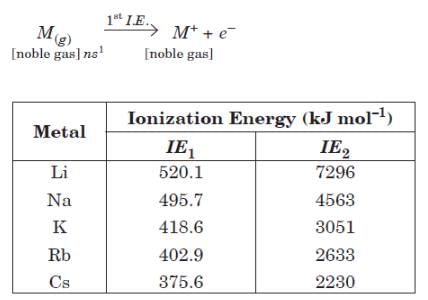
Q. Alkali metals displace hydrogen from water forming bases due to the reason that
Read the passage given below and answer the following questions.
Alkali metals have the lowest ionization energy in their corresponding period in periodic table because they have large size which results in a large distance between the nucleus and the outermost electron. Ionization energy of alkali metals decreases from Li to Cs due to increase in atomic size. First ionization energy of alkali metals is very low, but they have very high value of second ionization energy.

Q. Metals dissolve in liquid ammonia giving coloured solutions which are conducting in nature. The colour of the solution and reason of its conductance is
The lighter alkaline earth metals are very important in animal and plant physiology. Magnesium functions as the active center in some enzymes and calcium salts take a structural role in bones. The alkaline earth metals have the second lowest first ionisation energies in their respective periods. The ions of these elements perform important biological functions like maintenance of ion balance and nerve impulse conduction. The given questions (i) to (iv) consist of an assertion (A) and reason (R). Choose the correct option.
Assertion: Alkaline earth metals are reducing agents.
Reason: They have low ionisation enthalpies and high negative values of E°.














