NCERT Based Test: Periodic Trends in Properties of Elements - NEET MCQ
30 Questions MCQ Test - NCERT Based Test: Periodic Trends in Properties of Elements
What are the two radii shown as 'a' and 'b' in the figure known as?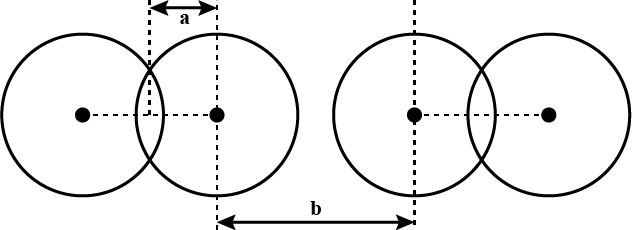

Few values are given in the table in the direction from left to right and top to bottom. Predict the property which could be depicted in the table.
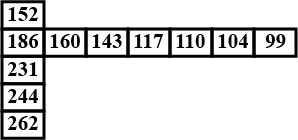
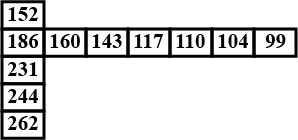
Which of the following statements regarding the variation of atomic radii in the periodic table is not true?
Ionic radius in a group while moving down.
K+ and Cl- ions are isoelectronic. Which of the statements is not correct?
Which of the following transitions will involve maximum amount of energy?
What is the order of successive ionisation enthalpies?
Ionisation enthalpy of nitrogen is more than oxygen because of
Which of the following elettients will have highest second ionisation enthalpy?
Which of the following can most easily form unipositive gaseous ion?
Of the metals Be, Mg., Ca and Sr of group 2 in the periodic table, the least ionic chloride will be formed by
A sudden large jump between the values of second and third ionization energies of an element would be associated with which ofthe following electronic configuration?
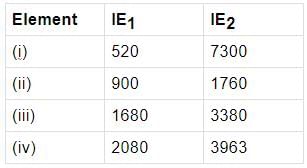
Few elements are matched with their successive ionisation energies. Identify the elements.
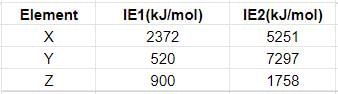
First and second ionisation enthalpies (in kj/mol) of few elements are given below:
Which of the above elements will form halides with formula MX2?
The electronic states X and Y of an atom are depicted below:
X : 1s2 2s2 2p6 3s1
Y : 1s2 2s2 2p6 3s2 3p6 4s1
Which of the following statements is not correct?
Which of the following elements will have highest ionisation energy?
Few values of enthalpies are given below:
O = - 141 kJ mol-1 F = - 328 kJ mol-1
S = - 200 kJ mol-1 Cl = - 349 kJ mol-1
Wliat do these values show?
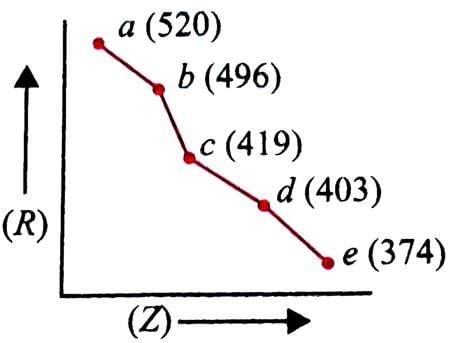
In the given graph, a periodic property (R) is plotted against atomic numbers (Z) of the elements. Which property is shown in the graph and how is it correlated with reactivity of the elements?
Which is correct increasing order of their tendency of the given elements to form M3- ion?
Which of the following will have lowest electron affinity?
Which of the following arrangements represents the correct order of electron gain enthalpy?
Why is the electron gain enthalpy of O or F less than that of S or Cl?
Which of the foEowing statements regarding an anion is not true?
Which of the properties of isotopes of an element is different?
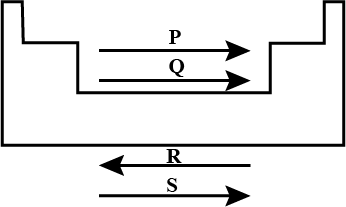
Given below are the names of few elements based on their position in the periodic table. Identify the element which is not correctly placed.
Why do noble gases have positive electron gain enthalpy?
As we move from left to right, the electronegativity increases. An atom which is highly electronegative has
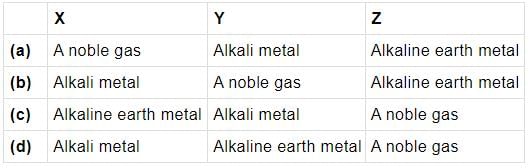
Study the given diagram and fill up the blanks with appropriate choice.
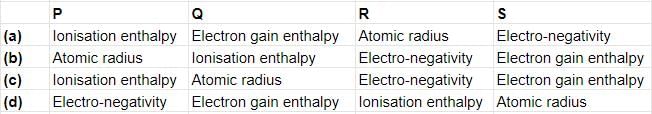
Fill in the blanks with the appropriate option.
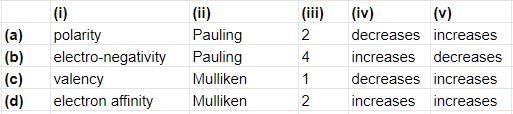
The ability of an atom to attract shared electrons to itself is called (i). It is generally measured on the (ii) scale. An arbitrary value of (iii) is assigned to fluorine (have greatest ability to attract electrons). It generally (iv) across a period and (v) down a group.
In which of the following, the order is not in accordance with the property mentioned.















