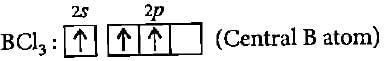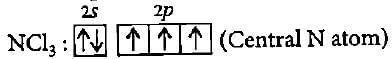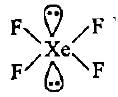Test: The Valence Shell Electron Pair Repulsion Theory (NCERT) - NEET MCQ
15 Questions MCQ Test - Test: The Valence Shell Electron Pair Repulsion Theory (NCERT)
According to VSEPR theory
In a bonded molecule, the order of repulsion between the bonded and non-bonded electrons is
The shape of water molecule which should be tetrahedral has a bent or distorted tetrahedral shape with a bond angle 104.5°. What could be the reason for this?
Which of the following shapes of SF4 is more stable and why?
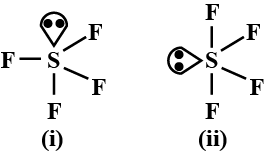
The most stable shape of ClF3 is shown by
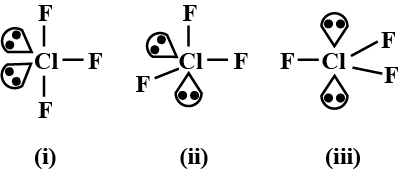
In which of the following molecules the central atomdoes not retain any lone pair of electrons?
Few examples of the compounds formed by chemical bonding are given below. Mark the incorrect example.
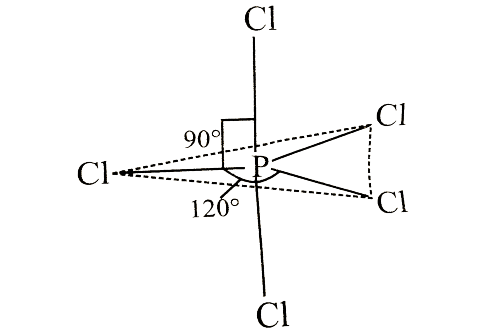
Which of the following statements is correct regarding the structure of PCI5?
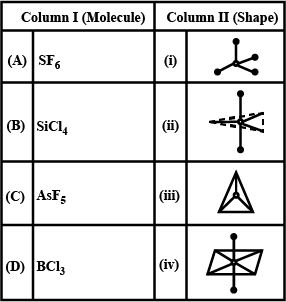
Match the molecules given in column I with their shapes given in column II and mark the appropriate choice
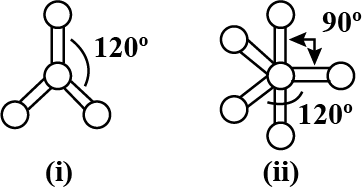
What is common between the following molecules
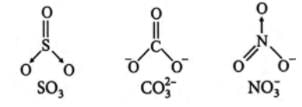
SO3, CO2-3, No-3
Which molecule is depicted by the given ball and stick models?
Which of the following does not show octahedral geometry?
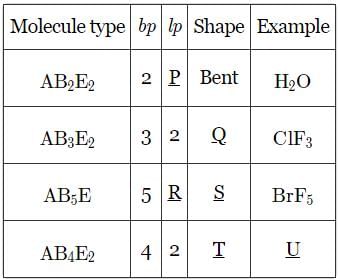
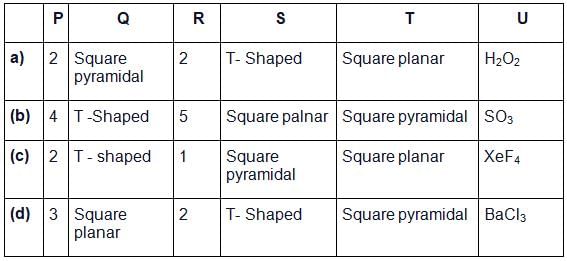
Given below is the table showing shapes of some molecules having lone pairs of electrons. Fill up the blanks left in it.
The BCI3 is a planar molecule whereas NCI3 is pyramidal, because
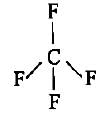
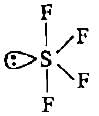
CF4, SF4 and XeF4 contain the following electronicstructure on their central atoms. Which one iscorrect option?