Test: Adsorption (Old NCERT) - NEET MCQ
25 Questions MCQ Test - Test: Adsorption (Old NCERT)
After the reaction is over between adsorbed reactants, it is important to create space for the other reactant molecules to approach the surface and react. The process responsible for this is known as
Which of the following statements does not show correct difference between adsorption and adsorption?
Powdered substances are more effective adsorbents than their crystalline form because
Which of the following is less than zero during adsorption?
Which of the following is a property of physisorption?
Which of the following statements is not correct about physisorption?
Adsorption is accompanied by the evolution of heat. So according to Le-Chatelier principle, the amount of substance adsorbed should
Which of the following gases is least adsorbed on charcoal?
Which of the following is not characteristic of chemisorpiton?
Chemisorption involves formation of bond between gaseous molecules or atoms and the solid surface for which high energy is required. Thus it is also referred as
Which of the following is not correct regarding the adsorption of a gas on the surface of solid?
Which of the plots is adsorption isobar for chemisorption?
Which of the following statements is not correct for chemisorption and physisorption?
In the adsorption of a gas on solid, Freundlich isotherm is obeyed. The slope of the plot is zero. Thus, the extent of adsorption is
In Freundlich adsorption equation x/m = kp1/n, the value of n is
Which of the following graphs would yield a straight line?
A graph is plotted between log (x/m) and P according to the equation x/m = kp1/n

Which of the following statements about this graph is not correct?
At low pressure, the fraction of the surface covered follows
What is the role of activated charcoal in gas masks used in mines?
Which of the following gases present in a polluted area will be adsorbed most easily on the charcoal gas mask?
Match the column I with column II and mark the appropriate choice.
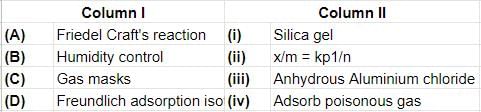
Which of the following is application of adsorption in chemical analysis?




















