Test: Solubility (NCERT) - NEET MCQ
10 Questions MCQ Test - Test: Solubility (NCERT)
During dissolution when solute is added to the solvent, some solute particles separate out from the solution as a result of crystallisation. At the stage of equilibrium, the concentration of solute in the solution at given temperature and pressure
Consider the two figures given below.
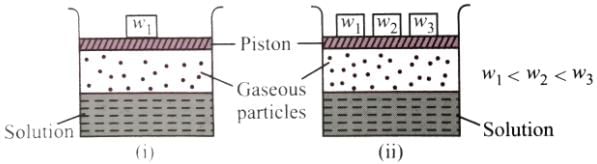
Which of the following statements regarding the experiment is true?

Which of the following statements regarding the experiment is true?
The law which indicates the relationship between solubility of a gas in liquid and pressure is________.
According to Henry's law the partial pressure of the gas in vapour phase (p) is proportional to the mole fraction of the gas (x) in the solution. For different gases the correct statement about Henry's constant is
The value of Henry's law constant for some gases at 293K is given below. Arrange the gases in the increasing order of their solubility.
He = 144.97kbar; H2 = 69.16kbar
N2 = 76.48kbar; O2 = 34.86kbar
H2S is a toxic gas used in qualitative analysis. If solubility of H2S in water at STPSTP is 0.195m, what is the value of KH?
Henry's law constant for the molality of methane in benzene at 298 K is 4.27 × 105 mm Hg. The mole fraction of methane in benzene at 298 K under 760 mm Hg is
When a gas is bubbled through water at 298K, a very dilute solution of gas is obtained. Henry’s law constant for the gas is 100kbar. If gas exerts a pressure of 1bar1bar, the number of moles of gas dissolved in 11 litre of water is
How much oxygen is dissolved in 100mL water at 298K if partial pressure of oxygen is 0.5atm and KH = 1.4 × 10−3 mol / L / atm?
At high altitudes the partial pressure of oxygen is less than that at the ground level. This leads to




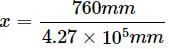 = 1.78 x 10-3
= 1.78 x 10-3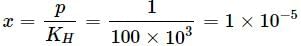
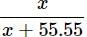 (55.55 >>> x)
(55.55 >>> x)