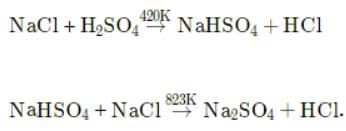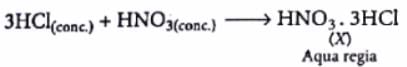NEET Exam > NEET Tests > Test: Chlorine & Hydrogen Chloride - NEET MCQ
Test: Chlorine & Hydrogen Chloride - NEET MCQ
Test Description
5 Questions MCQ Test - Test: Chlorine & Hydrogen Chloride
Test: Chlorine & Hydrogen Chloride for NEET 2025 is part of NEET preparation. The Test: Chlorine & Hydrogen Chloride questions and answers have been prepared
according to the NEET exam syllabus.The Test: Chlorine & Hydrogen Chloride MCQs are made for NEET 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Chlorine & Hydrogen Chloride below.
Solutions of Test: Chlorine & Hydrogen Chloride questions in English are available as part of our course for NEET & Test: Chlorine & Hydrogen Chloride solutions in
Hindi for NEET course.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free. Attempt Test: Chlorine & Hydrogen Chloride | 5 questions in 5 minutes | Mock test for NEET preparation | Free important questions MCQ to study for NEET Exam | Download free PDF with solutions
Test: Chlorine & Hydrogen Chloride - Question 1
Which of the following is used to prepare Cl2 gas at room temperature from concentrated HCl?
Detailed Solution for Test: Chlorine & Hydrogen Chloride - Question 1
Test: Chlorine & Hydrogen Chloride - Question 2
If chlorine is passed through a solution of hydrogen sulphide in water, the solution turns turbid due to the formation of:
Detailed Solution for Test: Chlorine & Hydrogen Chloride - Question 2
Test: Chlorine & Hydrogen Chloride - Question 3
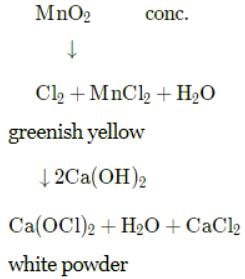
A black powder when heated with conc. HCl gives a greenish yellow gas. The gas acts as an oxidizing and a bleaching agent. When it is passed over slaked lime, a white powder is formed which is a ready source of gas. The black powder and white powder respectively are:
Detailed Solution for Test: Chlorine & Hydrogen Chloride - Question 3
Detailed Solution for Test: Chlorine & Hydrogen Chloride - Question 4
Test: Chlorine & Hydrogen Chloride - Question 5
When three parts of conc. HCl and one part of conc. HNO3 is mixed, a compound 'X' is formed. The correct option related to 'X' is:
Detailed Solution for Test: Chlorine & Hydrogen Chloride - Question 5
Information about Test: Chlorine & Hydrogen Chloride Page
In this test you can find the Exam questions for Test: Chlorine & Hydrogen Chloride solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Chlorine & Hydrogen Chloride, EduRev gives you an ample number of Online tests for practice
Download as PDF