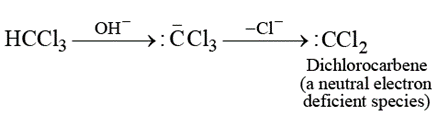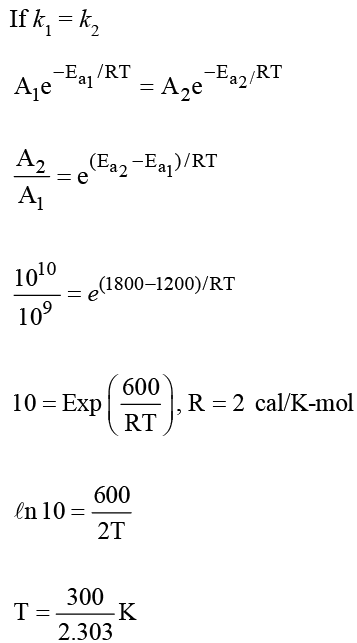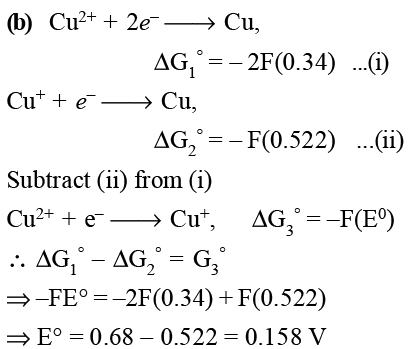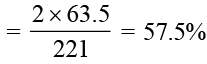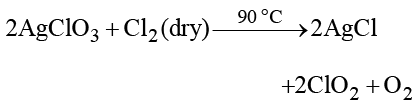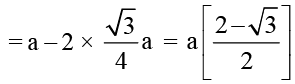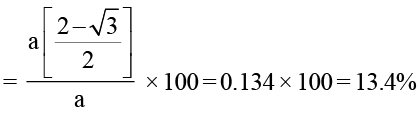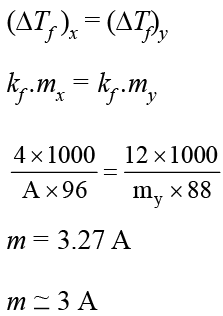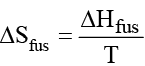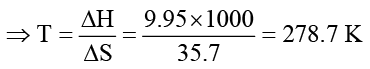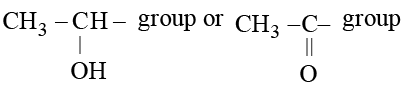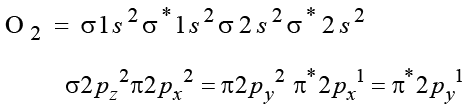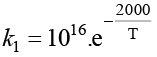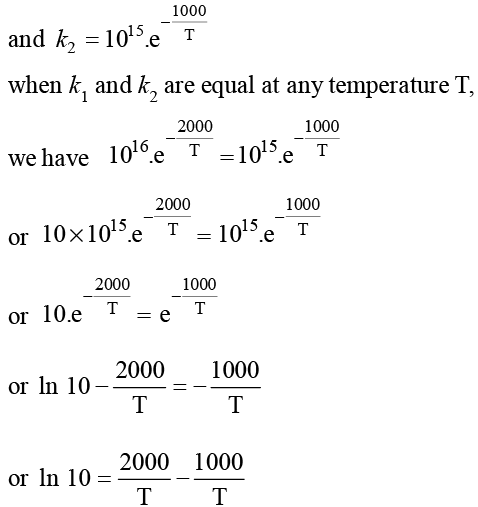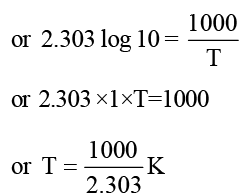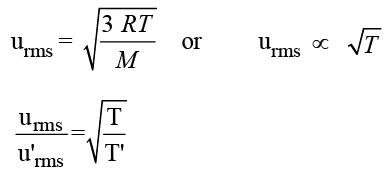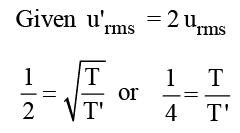NEET Practice Test - 13 - NEET MCQ
30 Questions MCQ Test - NEET Practice Test - 13
Reaction of ethyl amine with alkaline chloroform leads to the formation of carbylamine reaction. This reaction involves the attack of an electrophile on ethyl amine, the electrophile is
Although CN– ion and N2 molecule are isoelectronic, yet N2 molecule is chemically inert because of
For reaction A → B, the rate constant 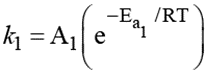 and for the reaction X → Y, the rate constant k2 = A2 (e-Ea2/RT). If A1 = 109, A2 = 1010 and Ea1 = 1200 cal/mol and Ea 2 = 1800 cal/mol, then the temperature at which k1 = k2 is : (Given; R = 2 cal/Kmol)
and for the reaction X → Y, the rate constant k2 = A2 (e-Ea2/RT). If A1 = 109, A2 = 1010 and Ea1 = 1200 cal/mol and Ea 2 = 1800 cal/mol, then the temperature at which k1 = k2 is : (Given; R = 2 cal/Kmol)
A square planar complex is formed by hybridisation of which atomic orbitals?
Given that the standard potentials (E0) of Cu2 + /Cu and Cu+/Cu are 0.34 V and 0.522 V respectively, the E0 of Cu2 +/Cu + is:
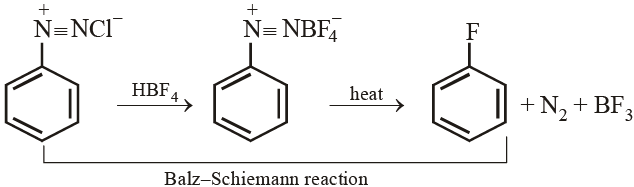
Aryl fluoride may be prepared from arene diazonium chloride using :
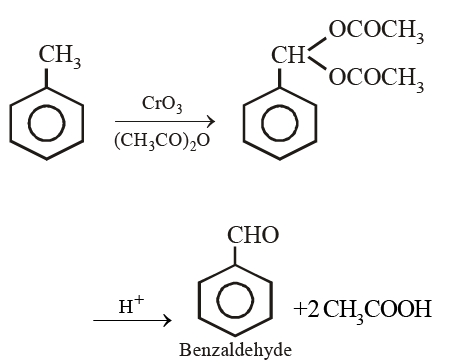
Toluene on treatment with CrO3 and (CH3CO)2O followed by hydrolysis with dil.HCl gives
Consider the equation Z = PV/RT. Which of the following statements is correct?
Malachite has the formula Cu2CO3(OH)2. What percentage by mass of malachite is copper?
Which one of the following oxides of chlorine is obtained by passing dry chlorine over silver chlorate at 90 °C?
A metal crystallises in the bcc lattice. The percent fraction of edge length not covered by atom is
Freezing point of a 4% aqueous solution of X is equal to the freezing point of the 12% aqueous solution of Y. If molecular weight of X is A, then molecular weight of Y is:
What is the melting point of benzene if ΔH fus = 9.95 kJ/mol and ΔS fus = 35.7 J/K mol?
Which of the following compounds cannot be used in preparation of iodoform?
The rate constants k1 and k2 for two different reactions are 1016. e–2000/T and 1015. e–1000/T, respectively. The temperature at which k1 = k2 is:
During nitration of benzene with nitrating mixture, HNO3 acts as
The root mean square velocity of a gas is doubled when the temperature is :
Of the interhalogen AX3 compounds, ClF3 is most reactive but BrF3 has higher conductance in liquid state. This is because