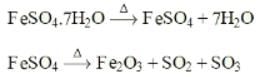Science Olympiad Test: Chemical Reactions and Equations- 1 - Class 10 MCQ
10 Questions MCQ Test - Science Olympiad Test: Chemical Reactions and Equations- 1
Which of the following is not a physical change?
When ferrous sulphate crystals are strongly heated, the gas/vapour not evolved are of
What is the corresponding change in colour when iron nail is dipped in the blue copper sulphate solution?
Which of the following statements about the given reaction are correct?
3 Fe(s) + 4 H2 O(g) → Fe3O4 (s) + 4 H2(g)
(i) Iron metal is getting oxidised
(ii) Water is getting reduced
(iii) Water is acting as reducing agent
(iv) Water is acting as oxidising agent.
The process of coating iron with zinc is called
Which of the following gases can be used for storage of fresh sample of an oil for a long time?
Which of the following metal will react with dilute hydrochloric acid to give out hydrogen gas
A dilute ferrous sulphate solution was added to the beaker containing acidified permanganate solution. The light purple colour of the solution fades and finally disappears. Which of the following is the correct explanation for the observation?