Class 10 Exam > Class 10 Tests > Science Olympiad Test: Carbon and Its Compounds- 1 - Class 10 MCQ
Science Olympiad Test: Carbon and Its Compounds- 1 - Class 10 MCQ
Test Description
10 Questions MCQ Test - Science Olympiad Test: Carbon and Its Compounds- 1
Science Olympiad Test: Carbon and Its Compounds- 1 for Class 10 2025 is part of Class 10 preparation. The Science Olympiad Test: Carbon and Its Compounds- 1 questions and answers have been prepared
according to the Class 10 exam syllabus.The Science Olympiad Test: Carbon and Its Compounds- 1 MCQs are made for Class 10 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Science Olympiad Test: Carbon and Its Compounds- 1 below.
Solutions of Science Olympiad Test: Carbon and Its Compounds- 1 questions in English are available as part of our course for Class 10 & Science Olympiad Test: Carbon and Its Compounds- 1 solutions in
Hindi for Class 10 course.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free. Attempt Science Olympiad Test: Carbon and Its Compounds- 1 | 10 questions in 10 minutes | Mock test for Class 10 preparation | Free important questions MCQ to study for Class 10 Exam | Download free PDF with solutions
Science Olympiad Test: Carbon and Its Compounds- 1 - Question 1
Carbon forms a large member of organic compounds due to
Detailed Solution for Science Olympiad Test: Carbon and Its Compounds- 1 - Question 1
Science Olympiad Test: Carbon and Its Compounds- 1 - Question 2
Ethane, with molecular formula C2H6 has
Detailed Solution for Science Olympiad Test: Carbon and Its Compounds- 1 - Question 2
Science Olympiad Test: Carbon and Its Compounds- 1 - Question 3
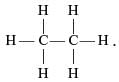
Which of the following are correct structural isomers of butane?
(i)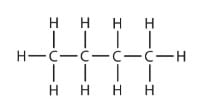
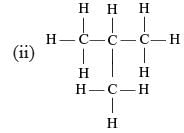
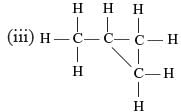
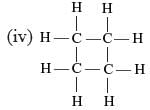
(i)




Detailed Solution for Science Olympiad Test: Carbon and Its Compounds- 1 - Question 3
Science Olympiad Test: Carbon and Its Compounds- 1 - Question 4
Functional groups present in aspirin
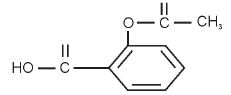
Detailed Solution for Science Olympiad Test: Carbon and Its Compounds- 1 - Question 4
Science Olympiad Test: Carbon and Its Compounds- 1 - Question 5
Buckminster fullerene is an allotropic form of
Detailed Solution for Science Olympiad Test: Carbon and Its Compounds- 1 - Question 5
Science Olympiad Test: Carbon and Its Compounds- 1 - Question 6
Which of the following substances is added to denature ethanol?
Detailed Solution for Science Olympiad Test: Carbon and Its Compounds- 1 - Question 6
Science Olympiad Test: Carbon and Its Compounds- 1 - Question 7
A molecule of ammonia (NH3) has
Detailed Solution for Science Olympiad Test: Carbon and Its Compounds- 1 - Question 7
Science Olympiad Test: Carbon and Its Compounds- 1 - Question 8
Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms, e.g., hydrogen. After the formation of four bonds, carbon attains the electronic configuration of
Detailed Solution for Science Olympiad Test: Carbon and Its Compounds- 1 - Question 8
Science Olympiad Test: Carbon and Its Compounds- 1 - Question 9
Which of the following set of compounds have the same molecular formula?
Detailed Solution for Science Olympiad Test: Carbon and Its Compounds- 1 - Question 9
Detailed Solution for Science Olympiad Test: Carbon and Its Compounds- 1 - Question 10
Information about Science Olympiad Test: Carbon and Its Compounds- 1 Page
In this test you can find the Exam questions for Science Olympiad Test: Carbon and Its Compounds- 1 solved & explained in the simplest way possible.
Besides giving Questions and answers for Science Olympiad Test: Carbon and Its Compounds- 1, EduRev gives you an ample number of Online tests for practice
Download as PDF