Science Olympiad Test: Acids, Bases and Salts- 1 - Class 10 MCQ
10 Questions MCQ Test - Science Olympiad Test: Acids, Bases and Salts- 1
Which of the following statements is true for acids?
When sodium hydroxide is added to ammonium carbonate salt and then a glass rod dipped in dilute hydrochloric acid is brought near the test tube, we observe
Which of the following phenomenon occur, when a small amount of acid is added to water?
(i) Ionisation
(ii) Neutralization
(iii) Dilution
(iv) Salt formation
What will be pH of solution when 0.02 mole of hydrochloric acid in 2 litres of the solution
Solutions A, B, C and D have pH 3, 4, 6 and 8. The solution with highest acidic strength is
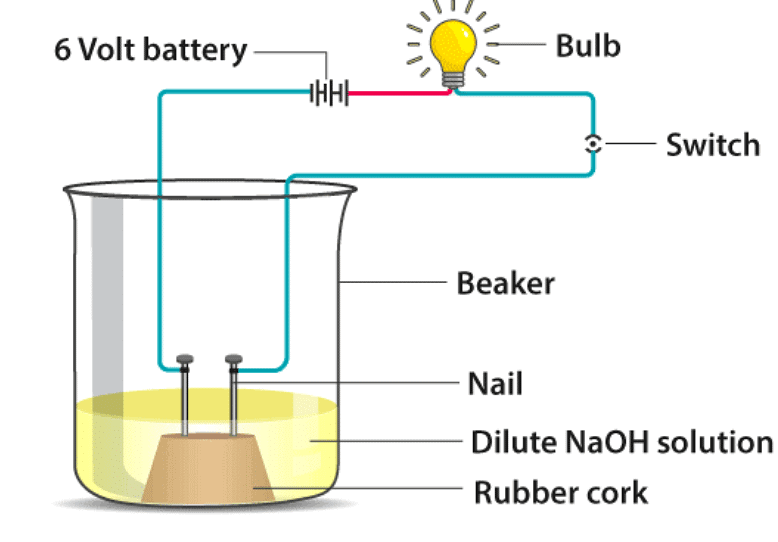
In an attempt to demonstrate electrical conductivity through an electrolyte, the following apparatus was set up
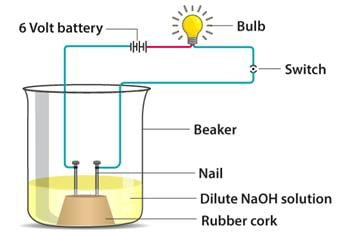
Which among the following statement(s) is (are) correct?
(i) Bulb will not glow because electrolyte is not acidic
(ii) Bulb will glow because NaOH is a strong base and furnishes ions for conduction
(iii) Bulb will not glow because circuit is incomplete
(iv) Bulb will glow because it does not depend on the type of solution
Which of the following is not a mineral acid?
Washing soda (Na2CO3.10 H2O) on exposure to air gives
Which of the following statements is not correct?