Olympiad Test: Matter in Our Surroundings- 3 - Class 9 MCQ
10 Questions MCQ Test - Olympiad Test: Matter in Our Surroundings- 3
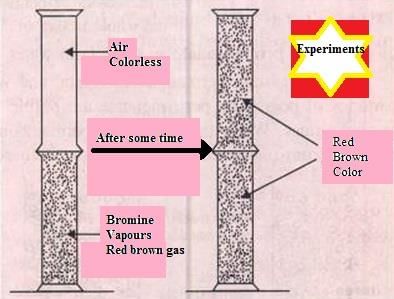
When a gas jar full of air is placed upside down on a gas jar full of bromine vapours, the red–brown vapours of bromine from the lower jar go upward into the jar containing air. In this experiment
Seema took a 100 mL beaker and filled half the beaker with water and marked the level of water.
She dissolved some salt with the help of a glass rod and recorded water level again. Choose the correct observation related to above activity
Which one of the following statements is not true?
Which of the following process/ processes release heat?
(i) Condensation (ii) Vaporisation
(iii) Freezing (iv) Melting
Which of the following factors are responsible for the change in state of solid carbon dioxide when kept exposed to air?
(i) Increase in pressure (ii) Increase in temperature
(iii) Decrease in pressure (iv) Decrease in temperature
One of the following does not undergo sublimation. This one is
Which of the following has minimum kinetic energy?
Convert the temperature of 300 k to the Celsius scale