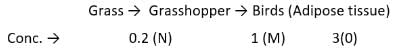National Level Solved Paper (SAT) - 2019-20 - Class 10 MCQ
24 Questions MCQ Test - National Level Solved Paper (SAT) - 2019-20
A taxonomist during his voyage found a solitary marine animal with spines on skin made of calcium carbonate. However, its coelom was made of pouches pinched off from the endoderm. Assign the specimen to the most appropriate Phylum.
An individual with genotype AaBbCcddEe is crossed with another individual with genotype AabbCcDdEe. Assuming the Mendelian pattern of inheritance, predict the proportion of aabbccddee among the progeny of this cross?
Which one of the four methods of propagation is likely to lead to maximum variation in DNA sequence through generations?
A case of bio-magnification was being studied. A laboratory received equal quantities of three samples M, N and O. The levels of pesticides found in these samples are as follows: M-1 mg, N-0.2 mg. O-3 mg.
The samples M, N and O respectively could be:
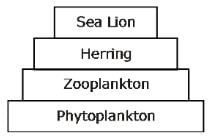
An illustration of a pyramid of a number of aquatic ecosystem is given below.
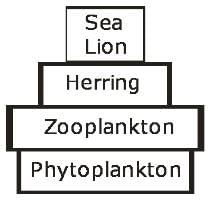
The pyramid of energy for the same ecosystem would be:
Which of the following traits would an evolutionary biologist consider to understand the divergent evolution process?
In adjacent agricultural lands of nearly equal dimensions, two farmers A and B had cultivated crops of their choice and observed standard practices. A pathogen attacked the crops and destroyed them in the land belonging to farmer A. as a result of which he suffered a complete loss. Although the pathogen attacked the adjacent land belonging to farmer B, he was able to earn some money by selling the yield. The possible explanation for the above is
A biology teacher placed a hen's egg in three different solutions:
Solution A: Pure water,
Solution B: saturated salt solution,
Solution C: Hydrochloric acid,
The sequence of treatments and the ensuing probable effect on the egg are listed below;
I. A → B → C → Remains unchanged
II. B → C → A → Swells
III. C → A → B → Shrinks
IV. B → A → C → Loses salts
Based on the above sequence to treatment which one of the options will be correct?
Observe the flow chart below.
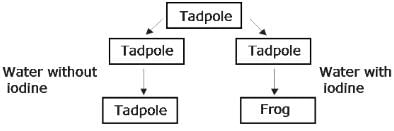
Which of the following best explains the observed results?
An experiment conducted in the laboratory is tabulated below.
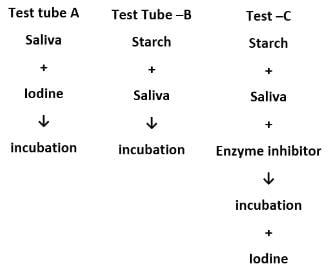
What would be the colour observed in test tubes A, B and C at the end of the experiment?
The presence of a specific molecule (called markers) in an organelle can be used to identify the presence of that organelle. A researcher has three test tubes with organelles. A, B and C, each of which shows the presence of one marker as shown below:

Based on the information given in the table, identify the organelles A, B and C.
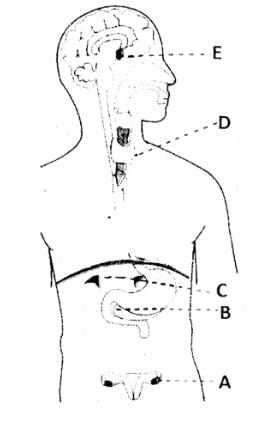
Positions of endocrine glands are labelled A – E in the given diagram. Match the symbols of glands in column 1 with the type of hormone it secretes given in column 2.
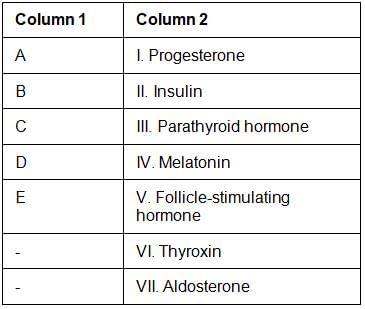
Choose the correct combination from the following:
Virulent forms of the bacterium staphylococcus aureus is a human pathogen. Some strains of which cause – 'flesh eating disease'. Earlier the antibiotic Penicillin was used to control this pathogen. After some years Penicillin was ineffective. Hence a powerful antibiotic- Methicillin was used in treatments. Subsequently, Methicillin also became ineffective and the strains showed resistance to multiple antibiotics also called "multi-drug resistance". Which one of the following statements regarding development of multi-drug resistance is correct?
1.80 g of glucose (C6H12O6) was dissolved in 36g of water. The number of oxygen atom in solution are:
Let T = Temperature, H = Humidity, and V = Wind speed
Which of the following are the best-suited conditions for drying up clothes?
100 mL of a solution containing 0.1 mole of NaOH per litre was mixed with 100 mL solution containing 0.02 mole of H2SO4 per litre. The amount of NaOH in the mixture in grams will be
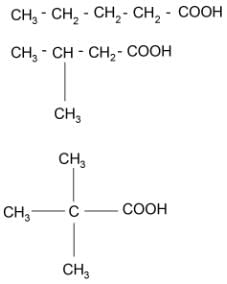
On oxidation with alkaline KMnO4 followed by acidification of the reaction mixture, which one of the following alcohols would produce an acid having three structural isomers?
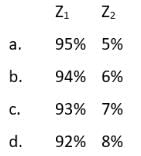
Atomic number of an element Z is 16. Element Z has two isotopes Z1 and Z2 with 16 and 18 neutrons, respectively. The average atomic mass of a sample of the element Z is 32.1 u. Which one of the following percentages of Z1 and Z2 in the sample is correct?
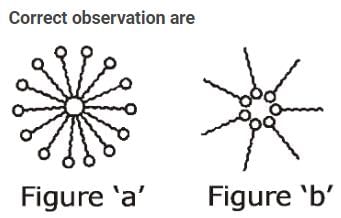
Detergents are also called surface-active agents (surfactants). These have two distinct parts: one hydrophilic spherical part and another hydrophobic long tail made of carbons chain. Two experiments ‘A’ and ‘B’ were carried out. In experiment ‘A’, surfactant was added in a beaker containing water. In experiment ‘B’, surfactant was added in a beaker containing hexane.
Following are possible results in these experiments:
I. In experiment ‘A’ (see figure ‘a’) micelle is formed, where the hydrophobic part is inside the micelle and the hydrophilic part is outside the micelle.
II. In experiment ‘B’ (see figure ‘b’) micelle of reverse type is formed where the hydrophilic part is inside the micelle and the hydrophobic part is outside the micelle.
III. Micelle of reverse type is formed in experiment ‘A’.
IV. Micelles are large enough to scatter light in ‘A’.
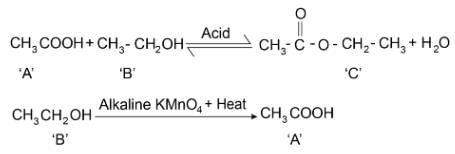
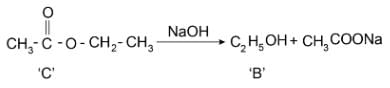
Reaction of organic compound ‘A’ with ‘B’ in acidic condition gives compound ‘C’. Compound ‘B’ reacts with alkaline KMnO4 solution and gives compound ‘A’. Compound ‘C’ gives compound ‘B’ as one of the product when reacted with sodium hydroxide solution. Which of the following statement is/are correct
I. ‘A’ is CH3COOH
II. ‘B’ is CH3CH2OH
III. ‘C’ is CH3COOCH2CH3
IV. ‘A’ is sweet-smelling substance
Equal volumes of solutions containing 1 mole of an acid and 1 mole of a base respectively are mixed. Which of these mixtures will give pH more than 7?
A part of the modern periodic table is shown below in which elements have been represented by English letters of the alphabet.
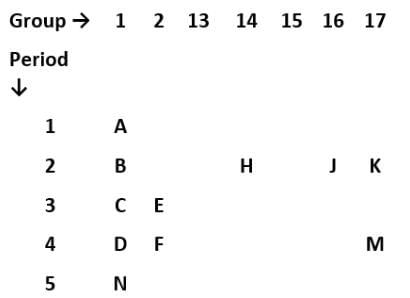
On the basis of the above periodic table, which one of the following statements is incorrect?
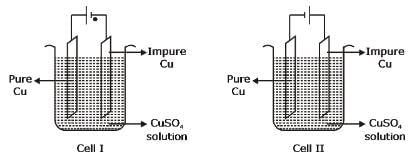
Consider the electrochemical cells (I and II) shown in the following figures and select the correct statement about these cells