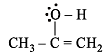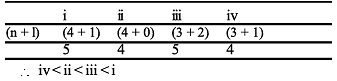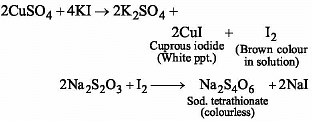NEET Practice Test - 16 - NEET MCQ
30 Questions MCQ Test - NEET Practice Test - 16
In an adiabatic process, no transfer of heat takes place between system and surroundings.
Choose the correct option for free expansion of an ideal gas under adiabatic condition from the following.
The correct order of solubility in water for He, Ne, Ar, Kr, Xe is
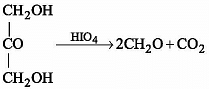
When dihydroxyacetone reacts with HIO4, the product is/are :
Which of the following possesses a sp-carbon in its structure ?
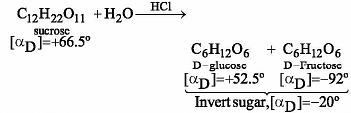
Sucrose in water is dextro-rotatory, [α]D= + 66.4º.
When boiled with dilute HCl, the solution becomes leavo-rotatory, [α]D = –20º. In this process the sucrose molecule breaks into
Which of the following statement is not true about secondary structure of protein ?
The tendency of BF3, BCl3 and BBr3 to behave as Lewis acid decreases in the sequence:
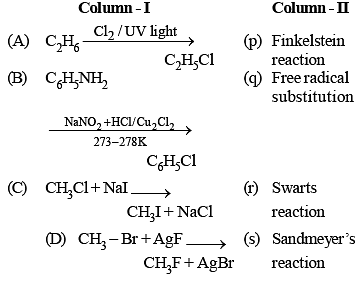
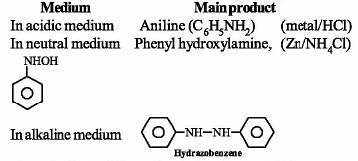
Which of the following reactions can produce aniline as main product?
Following reaction occurrs in an automobile
2C8H18 (g) + 25O2 (g) → 16CO2 (g) + 18H2O (g)
The sign of ΔH,ΔS and ΔG would be
Complexes formed in the following methods are
I. Mond's process for purification of nickel.
II. Removal of lead poisoning from the body.
III. Cyanide process for extraction of silver.
IV. Froth flotation process for separation of ZnS from galena ore by using depressant.
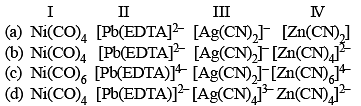
Which of the following does not represent the correct order of the properties indicated?
The electrons, identified by quantum numbers n and l
(i) n = 4, l = 1
(ii) n = 4, l = 0
(iii) n = 3, l = 2
(iv) n = 3, l = 1
can be placed in order of increasing energy, from the lowest to highest, as
In which of the following cases, the stability of two oxidation states is correctly represented
The major organic product in the reaction,
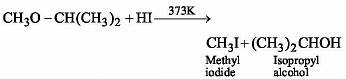
CH3 — O — CH(CH3)2 + HI → Product is
A certain compound (X) when treated with copper sulphate solution yields a brown precipitate. On adding hypo solution, the precipitate turns white. The compound is
Which one of the following complexes will have four different isomers ?