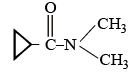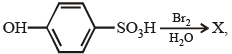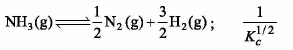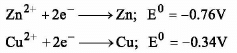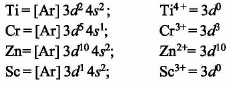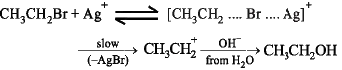NEET Practice Test - 17 - NEET MCQ
30 Questions MCQ Test - NEET Practice Test - 17
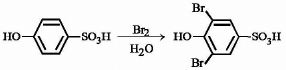
A is a lighter phenol and B is an aromatic carboxylic acid. Separation of a mixture of A and B can be carried out easily by using a solution of:
Which of the following compounds is a good conductor of electricity in solution state?
Which of the following is/are the hazardous pollutant(s) present in automobile exhaust gases?
(i) N2
(ii) CO
(iii) CH4
(iv) Oxides of nitrogen
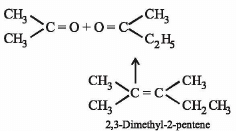
An alkene having molecular formula C7H14 was subjected to ozonolysis in the presence of zinc dust. An equimolar amount of the following two compounds was obtained
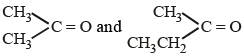
The IUPAC name of the alkene is
According to the adsorption theory of catalysis, the speed of the reaction increases because
The rate constant k, for the reaction
![]()
is 1.3 × 10–2s–1. Which equation given below describes the change of [N2O5] with time ? [N2O5]0 and [N2O5]t correspond to concentration of N2O5 initially and at time t.
An organic compound X on treatment with pyridinium chlorochromate in dichloromethane gives compound AND. Compound Y reacts with I2 and alkali to form triiodomethane. The compound 'X' is
(a)C2H5OH
Which is the major product formed when acetone is heated with iodine and potassium hydroxide?
The oxidation states of iodine in HIO4, H3IO5 and H5IO6 are respectively
Regarding F– and Cl– which of the following statements is/are correct?
(i) Cl– can give up an electron more easily than F–.
(ii) Cl– is a better reducing agent than F–.
(iii) Cl– is smaller in size than F–.
(iv)F– can be oxidized more readily than Cl–.
Among the following molecules
(i) XeO3
(ii) XeOF4
(iii) XeF6
Those having same number of lone pairs on Xe are
The value of Kc is 64 at 800 K for the reaction N2 (g) + 3H2 (g) ⇌ 2 NH3 (g) . The value of Kc for the following reaction is:
![]()
(I) n = 3, l = 2, m1 = – 2
(II) n = 3, l = 1, m1 = 0
(III) n = 3, l = 0, m1 = – 1
(IV) n = 3, l = 2, m1 = 0
(V) n = 3, l = 3, m1 = – 2
Of these question state designation which does not describe an allowed state for an electron in an atom?
Which is the most suitable reagent among the following to distinguish compound (3) from rest of the compounds ?
1. CH3 - C ≡ C - CH3
2. CH3 - CH2 - CH2 - CH3
3. CH3 - CH2C ≡ CH
4. CH3 - CH = CH2
Which of the following is spontaneous?
Zn2+ → Zn (s) ; E0 = - 0.76 V
Cu2+ → Cu (s) ; E0 = - 0.34 V
Which of the following complex ions is expected to absorb visible light?
(At. no. Zn = 30, Sc = 21, Ti = 22, Cr = 24)
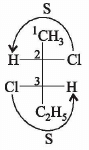
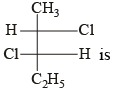
The absolute configuration of the following :
(b) The concentration of reactant molecules at the active centres of the catalyst becomes high due to strong adsorption
With which one of the following elements silicon should be doped so as to give a p-type of semiconductor ?
A sudden large jumps between the values of second and third ionization energies of an element, would be associated with which of the following electron configurations?
Ethanol can be prepared more easily by which reaction?
(i) CH3CH2Br + H2O → CH3CH2OH
(ii) CH3CH2 Br + Ag2O (in boiling water) → CH3CH2OH