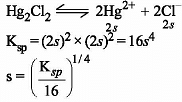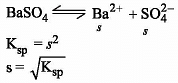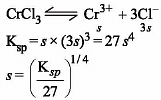NEET Practice Test - 24 - NEET MCQ
30 Questions MCQ Test - NEET Practice Test - 24
A compound of a metal ion Mx+ (Z = 24) has a spin only magnetic moment of √15 Bohr Magnetons. The number of unpaired electrons in the compound are
Iron sulphide is heated in air to form A, an oxide of sulphur. A is dissolved in water to give an acid. The basicity of this acid is
Which of the following is an expression of Raoult's law if PA is the partial pressure of the solvent in a solution. P0A is the partial pressure of pure solvent and if XA and XB are the mole fraction of the solute and the solvent respectively?
Phenol on reaction with Br2 in non-polar aprotic solvent furnishes
Which one of the following arrangements represents the correct order of solubilities of sparingly soluble salts Hg2Cl2, Cr2(SO4)3, BaSO4 and CrCl3 respectively ?
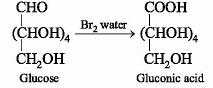
α-D-glucose and β-D-glucose differ from each other due to difference in one carbon with respect to its:
The hypothetical complex chloro diaquatriamminecobalt (III) chloride can be represented as
The pH of a solution is increased from 3 to 6; its H+ ion concentration will be
Identify the statement that is not correct as far as structure of diborane is concerned
Which one of the following compounds possesses the most acidic hydrogen?
Ozone is an important constituent of stratosphere because it:
Which one of the following is an example of homogeneous catalysis?
An element (X) forms compounds of the formula XCl3, X2O5 and Ca3X2 but does not form XCl5.
Which of the following is element X ?
In which of the following sets, all the given species are isostructural?
Which of the following cannot be made by using Williamson’s synthesis?
A metallic crystal crystallizes into a lattice containing a sequence of layers AB AB AB......Any packing of spheres leaves out voids in the lattice. What percentage of volume of this lattice is empty space?
Which among the following factors is the most important in making fluorine the strongest oxidizing halogen?
The most electropositive metals are isolated from their ores by:
The first ionization energies of alkaline earth metals are higher than those of the alkali metals.
This is because
The correct order of increasing acid strength of the compounds

Hydrogen peroxide acts both as an oxidising and as a reducing agent depending upon the nature of the reacting species. In which of the following cases H2O2 acts as a reducing agent in acid medium?