NEET Practice Test - 25 - NEET MCQ
30 Questions MCQ Test - NEET Practice Test - 25
The number of antibonding electron pairs inO2-2 molecular ions on the basis of molecular orbital theory are (at. no. O = 8)
The transition metals have a less tendency to form ions due to:
Brine is electrolysed by using inert electrodes.
The reaction at anode is ________.
According to the adsorption theory of catalysis, the speed of the reaction increases because
Consider the following complex [Co(NH3)5CO3]ClO4.The coordination number, oxidation number, number of d-electrons and number of unpaired d-electrons on the metal are respectively
The reducing power of divalent species decreases in the order
Beryllium shows a diagonal relationship with aluminium. Which of the following similarity is incorrect :
(n – 1)d10 ns2 is the general electronic configuration of
Which of the following crystals does not exhibit Frenkel defect?
Which of the following statements about low density polythene is FALSE?
Consider the following reaction :
![]()
(i) Rate of reaction with respect to NH 3 will be ![]()
(ii) For the given reaction ![]()
(iii) For the given reaction ![]()
(iv) For the given reaction, ![]() =
= ![]()
Which of the following are the correct statements.
Which of the following statements are correct ?
(i) Ionic product of water (Kw) = [H+] [OH–] = 10–14M2
(ii) At 298K [H+] = [OH–] = 10–7
(iii) Kw does not depends upon temperature
(iv) Molarity of pure water = 55.55M
Consider the following reaction at 1000°C
A. ![]()
B. ![]()
Choose the correct statement at 1000°C
Match List I (Reaction) with List II (Reagent) and select the correct answer using the codes given below the lists:

Values of dissociation constant, Ka are given as follows :
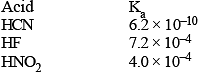
Correct order of increasing base strength of the base CN–, F– and NO2- will be :
Which of the following statements is not correct regarding aniline?
The de Broglie wavelength of a tennis ball of mass 60 g moving with a velocity of 10 m/s is approximately




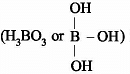 is a lewis acid so (b) is an incorrect statement.
is a lewis acid so (b) is an incorrect statement.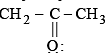 and
and 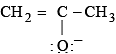 are
are
















