Test: VSEPR Theory & Hybridisation - JEE MCQ
26 Questions MCQ Test - Test: VSEPR Theory & Hybridisation
Direction (Q. Nos. 1-16) This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
The shape [CIF4]- and [ClF2]- ions is respectively
The shape [CIF4]- and [ClF2]- ions is respectively
Which of the following sequences shows the correct bond angle order for isoelectronic species 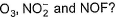
The species having pyramidal shape is
[IIT JEE 2010]
ECI3 (where, E = B, P, As, Bi) of these elements are known.
Bond angles are in the following order
Consider the two structures :
Select the correct statement(s).
Which of the two ions from the list given below that have the geometry that is explained by the same hybridisation of orbitals?
The correct order of increasing bond angles in the following species is
Which of the following molecule/species has the minimum number of lone pairs?
PCI5 has a shape of trigonal bipyramid whereas, IF5 has a shape of square pyramid. It is due to
For H2O and H2S, given
Q.
Difference in bond angle is due to
For which of the following sets of geometry, both axial and equatorial positions are present?
Which of the following set of molecules have the same shape but different hybridisation?
Direction (Q. Nos. 17-20) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE is correct.
Q.
Which of the following statements are correct regarding Cl2O molecule?
In AX4E2, atom A is surrounded by four atoms X and two lone pairs on it. This type of structure is predicted in
Which of the following sets of molecules have different shape but same hybridisation of the central atoms?
Direction (Q. Nos. 21-22) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).
Following reaction, CIF3 + AsF5 → (CIF2+ ) (AsF-6)
Q.
Select the correct statements.
Following reaction, CIF3 + AsF5 → (CIF2+ ) (AsF6-)
Q.
Select the correct statement(s).
Direction (Q. Nos. 23 and 24) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Q.
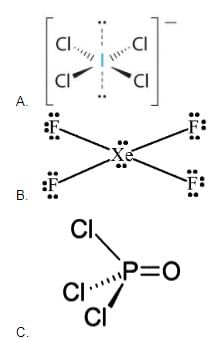
Match the species in Column I with the structure in Column II.
Match the species in Column I with the shape in Column II.
Direction (Q. Nos. 25 and 26) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive).
Q.
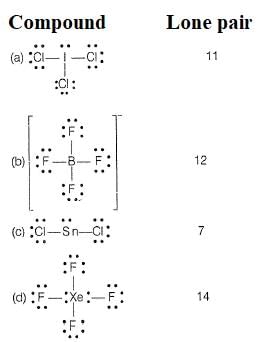
i. Based on VSEPR theory, the number of
ii. Based on VSEPR theory, number of lone pairs in XeF2 is......
iii. The total number of lone pair of electrons in melamine i s ......
[JEE Advanced 2013]
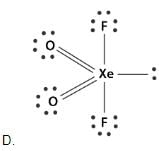
A list of species having the formula XZ4 is given below :
Defining the shape on the basis of location X and Z, the total number of species having a square planar shape is .......
[JEE Advanced 2014]