Test: Dipole Moment - JEE MCQ
19 Questions MCQ Test - Test: Dipole Moment
Direction (Q. Nos. 1-12) This section contains 12 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
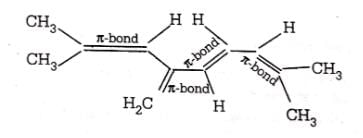
Number of π bonds in the following structure is–

Correct decreasing order of dipole moment of CH3F, CH3CI and CH3Br is
Consider the following compounds
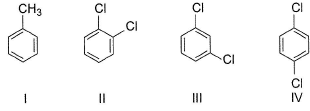
Q. Increasing order of dipole moment of these compounds is
Given for HCI and HI
Q. Ratio of fraction of electric charge existing on each atom in HCI and HI is
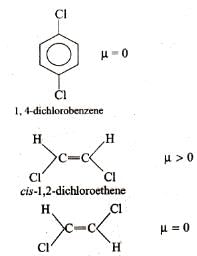
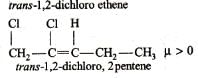
Ortho-isomers have dipole moments. In which cases dipole moments are maximum and minimum?
In which of the following sets of compounds dipole moment of I is larger than that of II?
Direction (Q. Nos. 13-15) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Which of the following has/have zero dipole moments?
Direction (Q. Nos. 16-17) This section contains a paragraph, each describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).
CH4 is subjected to photochemical reaction and changes to CH3CI, CH2CI2, CHCI3 and CCI4.
Q.
Select the correct alternate.
CH4 is subjected to photochemical reaction and changes to CH3CI, CH2CI2, CHCI3 and CCI4.
Q. One of the intermediates of the photochemical reaction is
Direction (Q. Nos. 18 and 19) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive).
Q. How many of the following molecules are polar?
Cl2, ICI, BF3, NO, SO2
XeF4, CH2CI2, OCS, CO2
How many molecules out of the following have non-zero value of dipole moments?
CH2CI2, CH4, CCI4, H2O, CHCI3, p-dichloro benzene, o-cresol, p-xylene, SCI2, BF3, IBr, HCHO