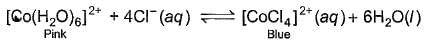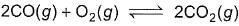Test: Chemical Equilibrium: Le Chatelier's Principle - NEET MCQ
19 Questions MCQ Test - Test: Chemical Equilibrium: Le Chatelier's Principle
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. The equilibrium which is not affected by volume change at constant temperature is
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
In each of the following equilibria, pressure is made four times after equilibrium is set up. In which case yield of the product(s) is maximum?
The binding of oxygen by haemoglobin (Hb) forming (HbO2), is partially regulated by the concentration of H3O + and dissolved CO2 in blood.
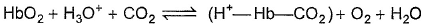
Release of O2 is favoured when there is
Aerated water contains CO2 dissolved in water
CO2(g) + H2O(l) H2CO3(aq)
H2CO3(aq)
Variation of solubility (s) with pressure (p) is shown by
Variation of log Kp with temperature. 1/T is given by for the equilibrium.
NH4HS (s) NH3(g) + H2S
Q. The equilibrium is displaced in forward side on
In the following equilibrium AB  A+ + B-
A+ + B-
AB is 10% dissociated, when [AB] = 1M
Q. What is per cent dissociation if 1 M AB is dissociated in the presence of 1 M A+?
When hydrochloric acid is added to cobalt (II) nitrate solution at room temperature, the following reaction takes place
Q. The solution is blue at room temperature. However, it turns pink when cooled in a freezing mixture. Based upon this information, which of the following expression is correct for the forward reaction?
Consider the following equilibrium in a closed container
N2O4 (g)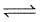 2NO2(g)
2NO2(g)
At a fixed temperature, the volume of a reaction container is halved. For this change, which of the following statements holds true regarding the equilibrium constant (Kp) and degree of dissociation (α) ?
Direction (Q. Nos. 9-18) This section contains 10 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
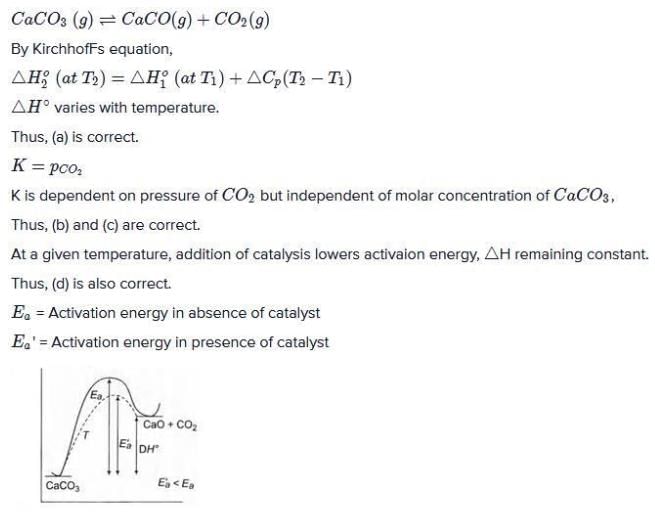
The thermal dissociation of equilibrium of CaCO3(s) is studied under different conditions
CaCO3 (s) 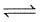 CaO(s) + CO2 (g)
CaO(s) + CO2 (g)
For this equilibrium, the correct statement (s) is/are
[JEE Advanced 2013]
For the reaction,
Equilibrium amount of CO2(g) can be increased by
Consider the following equilibrium,
N2(g) + 3H2(g)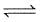 2NH3(g)
2NH3(g)
If N2(g) is added to the above mixture in equilibrium,
[IIT JEE 2006]
H2O (l) 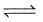 H2O(s) ; ΔH = -q
H2O(s) ; ΔH = -q
Application of pressure on this equilibrium
AgCI(s)is sparingly soluble salt,
AgCl (s) 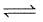 Ag+(aq) + Cl-(aq)
Ag+(aq) + Cl-(aq)
There is
Combustion of CO(g)can be increased in the following reaction by
2CO(g) +O2(g) 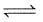 2CO2(g)
2CO2(g)
increase in pressure on the following equilibrium
H2O(l)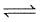 H20(g)
H20(g)
results is
Which of the following on the addition will cause deep red colour to disappear?
Sulphuric acid is manufactured by the following reaction
2SO2(g) +O2 (g) 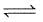 2SO3(g) +Q
2SO3(g) +Q
Reaction proceeds in the forward side if
For the reaction,
2SO2 (g) + O2 (g)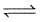 2SO3 (g) + 188.3 KJ
2SO3 (g) + 188.3 KJ
the number of moles of SO3 formed is increased if
We know that the relationship between Kc and Kp is Kp = Kc (RT)Δn
What would be the value of Δn for the reaction NH4Cl (s) ⇔ NH3 (g) + HCl (g)