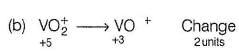Test: Oxidation Number in Redox Reaction - NEET MCQ
19 Questions MCQ Test - Test: Oxidation Number in Redox Reaction
Direction (Q. Nos. 1-12) This section contains 12 multiple-choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Consider the following reactions.
I. Zn + dil. H2So4 → ZnSO4 + H2
II. Zn + conc. H2SO4 → ZnSO4 + SO4 + H2O
III. Zn + HNO3 → Zn(NO3)2 + H2O + NH4NO3
Q. Oxidation number of H changes in:
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
I. Zn + dil. H2So4 → ZnSO4 + H2
II. Zn + conc. H2SO4 → ZnSO4 + SO4 + H2O
III. Zn + HNO3 → Zn(NO3)2 + H2O + NH4NO3
In which case change of oxidation number of V is maximum?
In which of the following set of compounds oxidation number of oxygen is not (- 2)?
Which of the following is not an example of redox reaction?
Which of the following atom has been assigned only single oxidation number?
The oxidation number of P in Ba(H2PO2)2, Ba(H2PO3)2 and Ba(H2PO4)2 are respectively
the oxidation number of sulphur in S8,S2F2,H2S respectively are
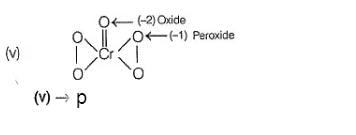
In which of the following reactions oxidation number of chromium has been affected?
Prussian blue is represented by KFex[Fe(CN)6]. Value of x is
In the following reaction, , oxidation number of
Select the set of compounds having fractional oxidation number in one or more atoms.
Direction (Q. Nos. 13 and 14) This section contains 2 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Which of the following species has/have oxidation number of the metal as + 6 ?
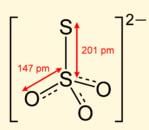
Oxidation numbers of P in PO4−3, of S in SO42− and that of Cr in Cr2O72− are respectively,
Direction (Q. Nos. 15-16) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)
The complex [Fe(H2O)5NO]2+ is formed in the ring-test for nitrate ion when freshly prepared FeSO4 solution is added to aqueous solution of
followed by the addition of conc. H2SO4. NO exists as NO+ (nitrosyl).
Q.Oxidation number of the Fe in the ring is
The complex [Fe(H2O)5NO]2+ is formed in the ring-test for nitrate ion when freshly prepared FeSO4 solution is added to aqueous solution of
followed by the addition of conc. H2SO4. NO exists as NO+ (nitrosyl).
Q. Magnetic moment of Fe in the ring is
Direction (Q. Nos. 17) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Match the compounds/ions having underlined atoms of different oxidation number (in Column I) with values (in Column II).
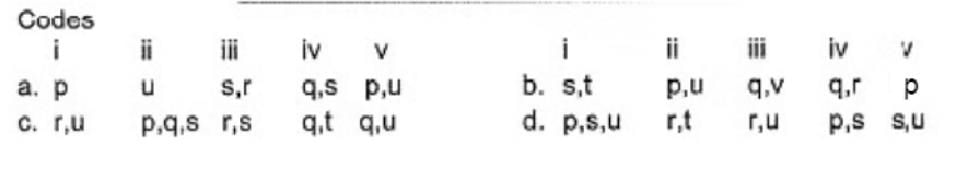

Direction (Q. Nos. 18 and 19) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive)
Q. S2O32- has two types of sulphur atoms. What is the difference in the oxidation states of two types of sulphur atoms?
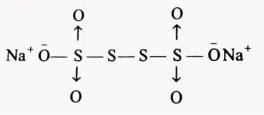
The difference in the oxidation number of the two types of sulphur atoms in Na2S4O6 is _________. [HTJEE2011]