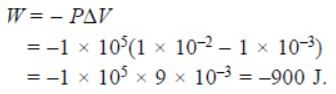Test: Thermodynamics: Basic Concepts - NEET MCQ
20 Questions MCQ Test - Test: Thermodynamics: Basic Concepts
Direction (Q. Nos. 1-15) This section contains 15 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
Assign the sign of work done (based on SI convention) in the following chemical changes taking place against external atmospheric pressure :
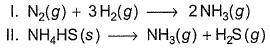
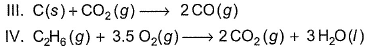
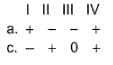
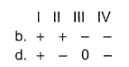
Assign the sign of work done (based on SI convention) in the following chemical changes taking place against external atmospheric pressure :
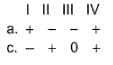
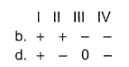
For the following process, H2 (g) → 2 H(g), it absorbs 436 kJ mol-1. Thus,
Suppose the elements X and Y combine to form two compounds XY2 and X3Y2. When 0.1 mole of XY2 weighs 10 g and 0.05 mole of X3Y2 weighs 9 g, the atomic weights of X and Y are
Five moles of an ideal gas at 27° C are allowed to expand isothermally from an initial pressure of 10.0 atm to a final pressure of 4.0 atm against a constant external pressure of 1.0 atm. Thus, work done will be
56 g of iron reacts with dilute H2SO4 at 27° C . W ork done (in cals) in
I. closed vessel of fixed volume and
II. an open vessel is


Consider the following properties.


Select intensive and extensive properties.
Consider the following properties.
State functions are
[IITJEE2009]
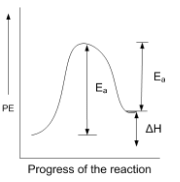
For an endothermic reaction when ΔH represents the enthalpy of the reaction in kJ mol-1, the minimum value for the energy of activation will be
[IIT JEE 1992]
One mole of an ideal gas is put through a series of changes as shown in the figure in which 1,2,3 mark the three stages of the system. Pressure at the stages 1, 2, and 3 respectively will be (in bar)


An ideal gas expands in volume from 1 × 10–3m3 to 1 × 10–2m3 at 300K against a constant pressure of 1 × 105Nm–2. The work done is
[AIEEE 2004]
One mole each of CaC2, AI4C3 and Mg2C3 reacts with H2O in separate open flasks at 25° C. Numerical value of the work done by the system is in order
Bond energy of (N— H)bond is x kJ mol-1 under standard state. Thus, change in internal energy in the following process is
Temperature of one mole of helium gas is increased by 2°. Thus, increase in internal energy is
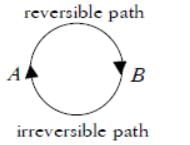
The internal energy change when a system goes from state A to B is 40kJ/mole. If the system goes from A to B by a reversible path and returns to state A by an irreversible path what would be the net change in internal energy?
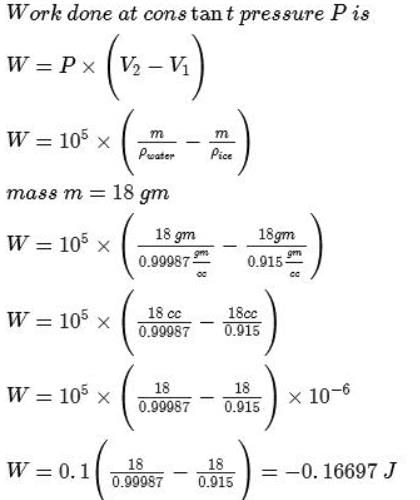
The density of ice at 0°C is 0.915 g cm-3 and that of liquid water at 0°C is 1.0 g cm-3. Work done for melting 1 mole of ice at 1.00 bar is
Direction (Q. Nos. 16 - 18) This section contains 3 questions. when worked out will result in an integer from 0 to 9 (both inclusive).
Q. Temperature of one mole of an ideal gas is increased by 1° at constant pressure. What is mechanical work done (in cals)?
A sample of gas changes from p1 V1 and T1, to p2, V2 and T2 by one path and then back to and T1, by another path. How many of the following must be zero for the gas in this cycle?
What will be the temperature change of 1.00 mole of CH3OH(g) if 100 J of heat is added to it under constant volume conditions?
Direction (Q. Nos. 19-20) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).
The following diagram represents the (p-V) changes of a gas.
Select isochoric changes
The following diagram represents the (p-V) changes of a gas.
Total work done is