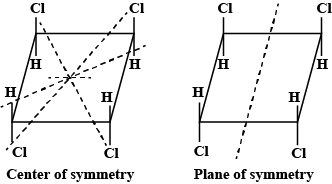
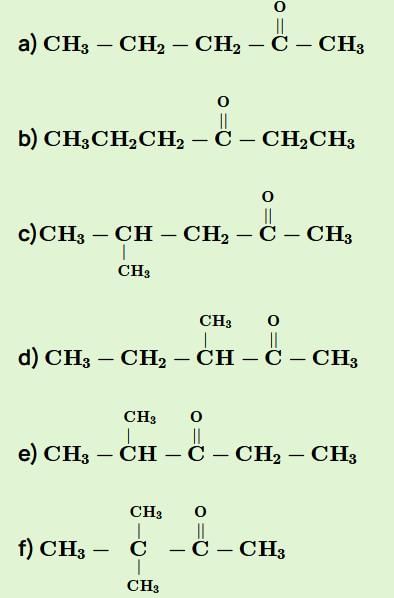Test: Optical Isomerism - JEE MCQ
20 Questions MCQ Test - Test: Optical Isomerism
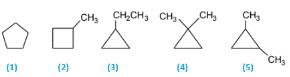
The number of optically active optical isomers of the compound is:
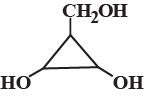

Find the number of stereoisomers for CH3 – CHOH – CH = CH – CH3.
A hydrocarbon X is optically. X upon hydrogenation gives an optically inactive alkane Y. Which of the following pair of compounds can be X and Y respectively?
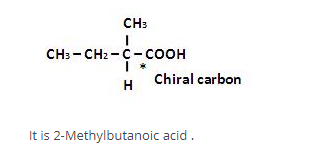
What is wrong about enantiomers of 2-chloropropanoic acid?
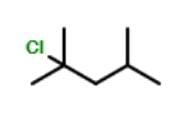
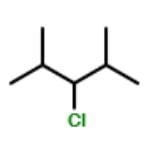
How many different stereoisomers exist for the compound below ?
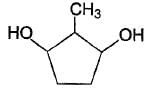
How the following pair of isomers cannot be distinguished ?
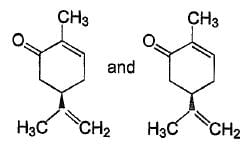
Consider all possible isomeric ketones, including stereoisomers of Molecular weight 100. All these isomers are independently reacted with NaBH4
(Note stereoisomers are also reacted separately). The total number of ketones that give a racemic product(s) is/are
Direction (Q. Nos. 10-14) This section contains 5 multiple choice questions. Each question has four
choices (a), (b), (c), and (d), out of which ONE or MORE THAN ONE is correct.
Q.Consider the following molecule
The correct statement concerning the above molecule is/are
Consider the following free radical chlorination reaction.
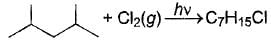
The correct statement about product(s) is/are
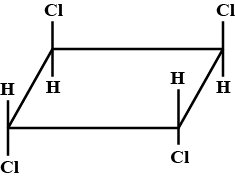
The correct statement(s) regarding Isomers of a compound with general name trichlorocyclobutane is/are
The correct statement (s) about the compound
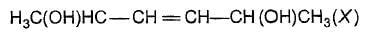
is/are
[IIT JEE 2009]
The correct statement(s) about the compound given below is/are
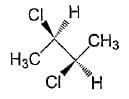
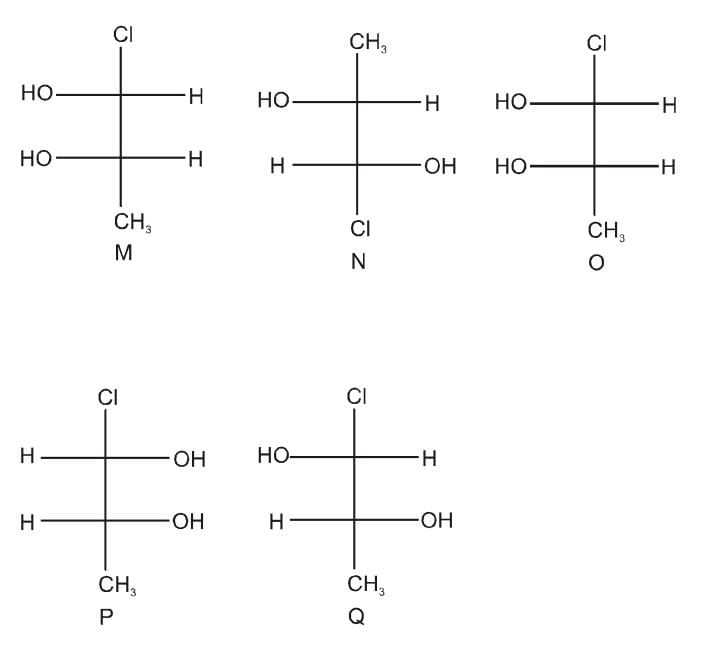
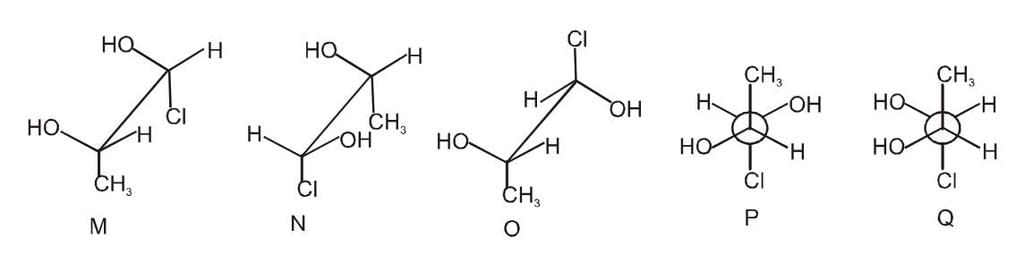
Which of the given statement (s) about N, O, P, and Q with respect to M is (are) correct?
The number of optical isomers possible for 2, 3-pentanediol is:
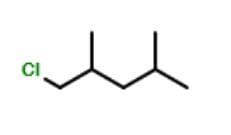
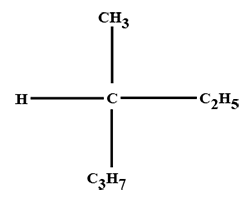
The minimum number of C atoms required for a hydrocarbon to exhibit optical isomerism:
Which of the following statements is/are correct regarding the below compound?
Which of the following are not functional isomers of each other?
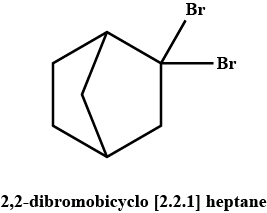
A pair of enantiomers is possible for _______ isomer of 2,2-dibromobicyclo [2.2.1] heptane.