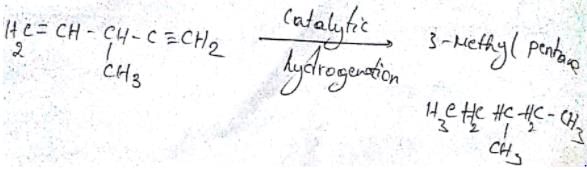Test: Stereoisomerism - JEE MCQ
21 Questions MCQ Test - Test: Stereoisomerism
Direction (Q. Nos. 1 - 6) This section contains 6 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
A pure enantiomer with molecular formula C6H13OBr, when reacted with PBr3, an achiral product C6H12Br2 is obtained that has no chiral carbon. The compound which satisfy this condition could be (no bond to chiral carbon is broken during the reaction)
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Optical rotation of a newly synthesised chiral compound is found to be +60°. Which of the following experiment can be performed to establish that optical rotation is not actually -300°?
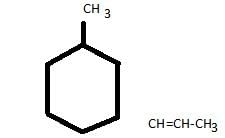
How many stereoisomers exist for the compound 4-(1- propenyl) cyclohexane ?
what is incorrect regarding cis -1, 3-dibromo - trans-2,4-dichlorocyclobutane ?
consider the following zonolysis reaction
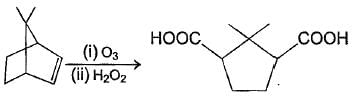
The correct statement about the above product formed is
A hydrocarbon with molecular formula C6H8 is chiral but upon catalytic hydrogenation gives achiral hydrocarbon of molar mass 86. Which of the following could be the starting compound?
Direction (Q. Nos. 7 and 14) This section contains 8 questions. when worked out will result in an integer from 0 to 9 (both inclusive)
Q.
How many stereoisomers exist in the compound 1-methyl-3-(1-propenyl) cyclohexane ?
If 3-ethylpentane is subjected to free radical chlorination, how many different monochlorination product would be produced?
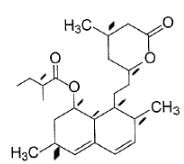
How many chiral carbons are present in the compound shown below ?
How many pairs of diastereomers can be made for the compound shown below ?
In principle, how many different monocarboxylic isomers, on decarboxylation with soda lime, can give the same 3-methyl pentane?
How many different stereoisomers exist for 1-chloro-2(3-chlorocyclobutyl) ethane?
How many different monoenols exist for 4-methyl-2,5-heptanedione ?
Direction (Q. Nos. 15-18) This sectionis based on statement I and Statement II. Select the correct answer from the code given below
Q.
Statement I A racemic mixture is optically inactive.
Statement II Racemic mixture contain a pair of enantiomers.
Q.
Statement I Cis-1,4-dichlorocyclobutane is optically inactive.
Statement II It possesses plane of symmetry.
Statement I 2,3-pentadiene is enantiomeric.
Statement II Enantiomers of 2,3-pentadiene are non-interconvertible due to very large rotational barrier.
Q.
Statement I : The Compound shown below is optically inactive.
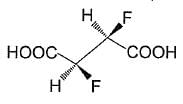
Statement II Compound shown above possesses axis of rotation.
Q.
Statement I Decreasing length of sample tube, while keeping everything else intact, decreases the magnitude of optical rotation.
Statement II Decreasing length of sample tube decreases the contribution of sample tube material to the total optical rotation.
Direction (Q. Nos. 20-21) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Q.
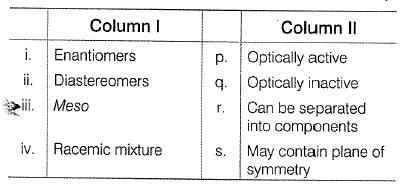
Consider the molecules in Column I and match them with their stereochemical properties from Column II

Match the stereochemical terms in column I with their description in column II