Test: Chemical Bonding and Shapes of Compounds - Chemistry MCQ
20 Questions MCQ Test - Test: Chemical Bonding and Shapes of Compounds
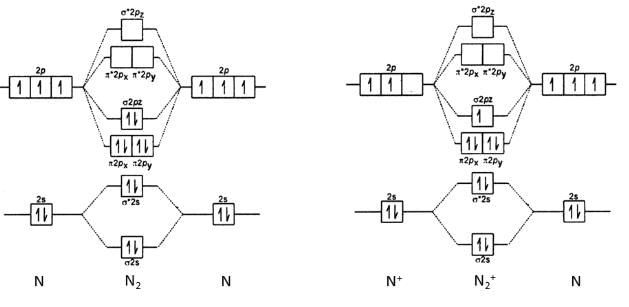
In the formation of N2+ from N2, the electron is removed from
During the formation of a molecular orbital from atomic orbitals, probability of electron density is
In the cyanide ion the negative charge is on
Amongst LiCl , RDCl , BeCl2 and MgCl2 the compounds with the greatest and the least ionic character, respectively are:
Which of the set of isomers of C6H4Cl2 is having equal dipole moment with C6H5Cl and C6H6 respectively
In ICl4Θ, the shape is square planar The number of bond pair-lone pair repulsion at 90° are:
Which of the following pairs is isostructural?
Which of the following statement is true?
A simplified application of MO theory to the hypothetical ‘molecule’ OF would give its bond order as :
Mg2C3 reacts with water forming propyne, C34- has:
Compound with maximum ionic character is formed fiom
The experimental value of the dipole moment of HCI is 1.03 D. The length of the H-CI bond is 1.275 A. The percentage of ionic character in HCl is
If a molecule MX3 has zero dipole moment, the sigma bonding orbitals used by M (atm. no. < 21) are
In which of the following molecule are all the bonds not equal ?
The cationic part of solid Cl2O6 is having the “_______ ” shape
The molecular shapes of SF4, CF4 and XeF4 are
In the context of carbon, which of the following is arranged in the correct order of electronegativity
A sigma bond may be formed by the overlap of 2 atomic orbitals of atoms A and B. If the bond is formed along the x-axis, which of the following overlaps is acceptable ?