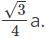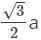Test: Packing Efficiency - Chemistry MCQ
10 Questions MCQ Test - Test: Packing Efficiency
If copper, density = 9.0 g/cm3 and atomic mass 63.5, bears face-centered unit cells then what is the ratio of surface area to volume of each copper atom?
How many atoms surround the central atom present in a unit cell with the least free space available?
“The packing efficiency can never be 100%”. Is this true or false?
If the body-centered unit cell is assumed to be a cube of edge length ‘a’ with spherical particles of radius ‘r’ then how is the diameter, d of particle and surface area, S of the cell related?
If metallic atoms of mass 197 and radius 166 pm are arranged in ABCABC fashion then what is the surface area of each unit cell?
What are the percentages of free space in a CCP and simple cubic lattice?
Which of the following metals would have the highest packing efficiency?
Arrange the types of arrangement in terms of decreasing packing efficiency.
What does the ratio ‘space occupied/total space’ denote?


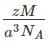
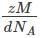
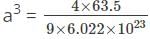
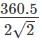 =127.46 pm
=127.46 pm