D And F Block Elements(Chapter Test - Non Medical) - Class 12 MCQ
30 Questions MCQ Test - D And F Block Elements(Chapter Test - Non Medical)
Which of the following is not a condition for complex salt formation ?
Match the Column I with Column II and mark the
appropriate choice
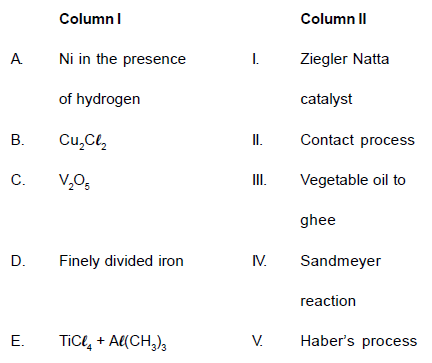

appropriate choice
The “spin-only” magnetic moment [in units of Bohr magneton, (μB)] of Fe2+ in aqueous solution would be
(At. No. Fe = 26)
(At. No. Fe = 26)
Which of the following reactions are not
disproportionation reactions ?
In chromite ore, the oxidation number of iron and chromium are respectively
Consider the following with respect to lanthanides
I. The basic strength of hydroxides of lanthanides increases from La(OH)3 to Lu(OH)3
II. The lanthanide ions Lu3+, Yb2+ and Ce4+ are diamagnetic Which of the following statement(s) given above is/are correct ?
KMnO4 acts as an oxidising agent in alkaline medium. When alkaline KMnO4 is treated with KI, iodide ion is oxidised to
Knowing that the chemistry of lanthanoids (Ln) is dominated by its +3 oxidation state, which of the
following statements is incorrect ?
When Hg2Cl2 ionizes, the ions produced and the unpaired electrons present on the cation respectively
are
Among the following, the compound that is both paramagnetic and coloured is
Four successive members of the first row transition elements are listed below with atomic numbers. Which
one of them is excepted to have the highest value ?
Which of the following statement is correct ?
Match the column I with column II and mark the appropriate choice.
Which of the following statements is false ?
When K2CrO4 is added to CuSO4 solution, there is formation of CuCrO4 as well as CuCr2O7. Formation of
CuCr2O7 is due to
A transition element M forms the oxides MO, M2O3, MO3 and M2O7. Which of the following statements about these oxides is true ?
If the lanthanoid element with fx electrons has a pink colour, then the lanthanoid with f14–x electrons will have the colour as
Which of the following statements is not correct ?
Identify the incorrect statementamong the following
The metallic radius of gold is almost identical with that of silver because of
Cerium can show the oxidation state of +4 because
When KMnO4 solution is added to oxalic acid solution, the decolourisation is slow in the beginning but
becomes instantaneous after some time because
Arrange the following in increasing the value of magnetic moments.
Which group contains coloured ions out of the following ?
Complete the following reactions
Match the column I with column II and mark the appropriate choice.
A violet compound of manganese (P) decomposes on heating to liberate oxygen and compounds (Q) and (R) of manganese are formed. Compound (R) reacts with KOH in the presence of potassium nitrate to give
compound (Q). On heating compound (R) with conc. H2SO4 and NaCl, chlorine gas is liberated and a
compound (S) of manganese along with other products is formed. Compounds P to S are
Which of the following reactions do not result in the preparation of potassium dichromate from chromate ?
Which of the following statements concerning lanthanides elements is false ?
Which of the following reactions is not correct ?



















