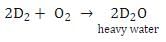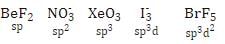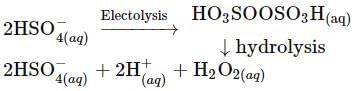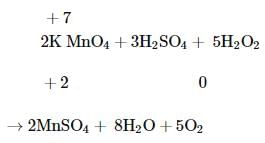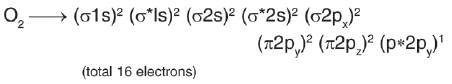BITSAT Chemistry Test - 3 - JEE MCQ
30 Questions MCQ Test - BITSAT Chemistry Test - 3
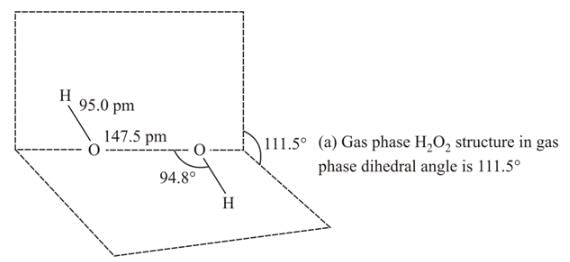
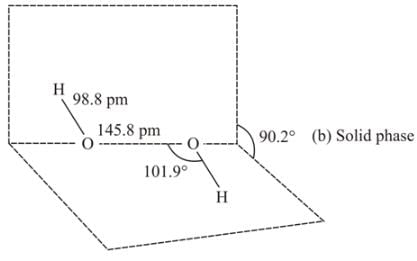
Hydrogen peroxide in its reaction with KIO4 and NH2OH respectively, is acting as a
There are three diatomic species with each having a bond order of three. What is true regarding them?
(a) All of them can be isoelectronic.
(b) They are isostructural.
(c) They may include cationic/anionic species.
(d) They may include neutral species.
HCl gas is covalent and NaCl is an ionic compound. This is because:
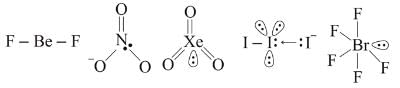
The correct order of decreasing s-character in hybrid orbitals among the following molecules
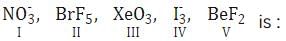
Which one of the following halides has the highest melting point?
What is the formula of a metal phosphate, if formula of its chloride is MCl 2 ?
The AsF5 molecule is trigonal bipyramidal. The hybrid orbitals used by the As atoms for bonding are
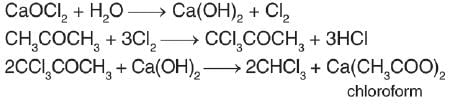
What is formed when calcium carbide reacts with heavy water?
Determine which of the following reaction would produce hydrogen gas (H2).
Which one of the following reactions does not form gaseous product ?
Dihydrogen of high purity (>99.95 %) is obtained through :
Hydrogen peroxide when added to a solution of potassium permanganate acidified with sulphuric acid
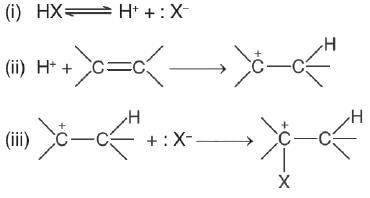
“The addition of unsymmetrical reagents to unsymmetrical alkenes occurs in such a way that the negative part of the addendum goes to that carbon atom of the double bond which carries lesser number of hydrogen atoms” is called by
Which of the following gas mixture is used by the divers inside the sea?