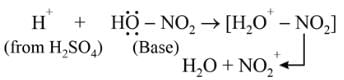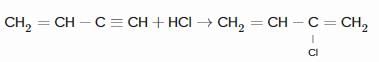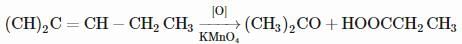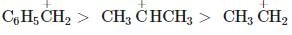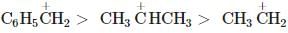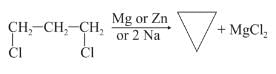BITSAT Chemistry Test - 4 - JEE MCQ
30 Questions MCQ Test - BITSAT Chemistry Test - 4
Which of the following group is sharp ortho and para directive?
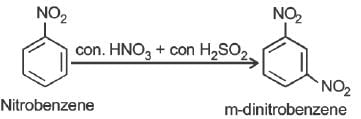
Nitrobenzene can be prepared from benzene by using a mixture of concentrated HNO3 and concentrated. H2SO4. In the mixture, nitric acid acts as a/an:
On vigorous oxidation by alkaline permanganate solution, (CH3)2C=CH−CH2CH3 gives:
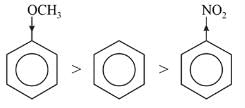
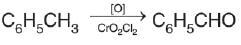
Among the following compounds (I-III) the correct order of reaction with electrophilic reagent is
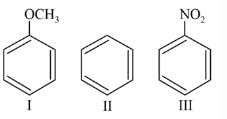
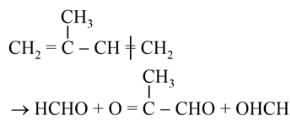
The products obtained on ozonolysis of the given alkene, followed by reaction with Zn/H2O are:
The number of geometrical isomers of CH3CH=CH−CH=CH−CH=CHCl is
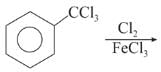
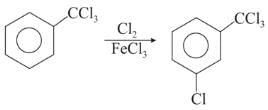
Chlorination of benzene is not possible in the following reaction.
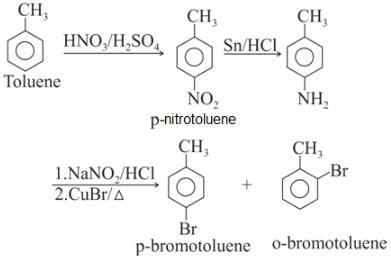
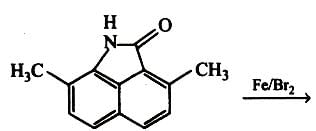
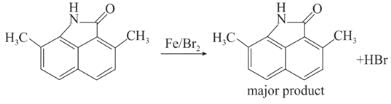
Toluene is nitrated and the resulting product is reduced with tin and hydrochloric acid. The product so obtained is mixed with NaNO2/HCl and CuBr and then heated, the product so formed contains.
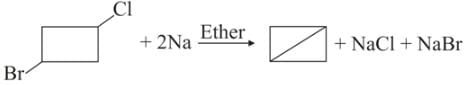
What would be the product formed when 1-bromo-3-chlorocyclobutane reacts with two equivalents of metallic sodium in ether?
Which of the following undergoes nucleophilic substitution exclusively by an SN1 mechanism
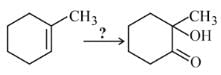
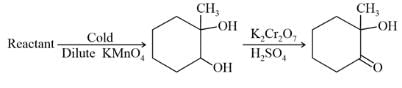
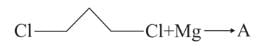
In the following sequence of reactions, 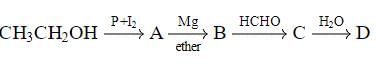
, then compound D is:
Which of the following compounds will form the precipitate with aq. AgNO3 solution most readily?
The specific rate constant of a first order reaction depends on
The law which explains the law of conservation of mass is
Two molecules of an ideal gas expand spontaneously into a vacuum. The work done is
At 25°C the pH value of a solution is 6, the solution is