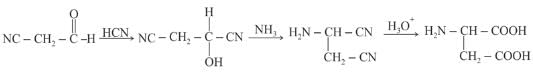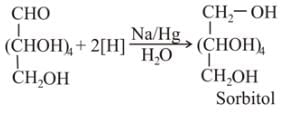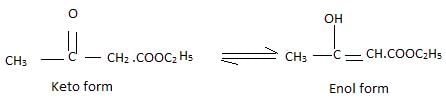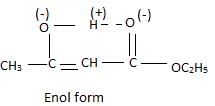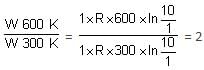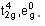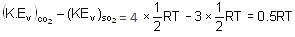BITSAT Chemistry Test - 8 - JEE MCQ
30 Questions MCQ Test - BITSAT Chemistry Test - 8
In towns, a layer of air is condensed as smoke due to pollution. It is called
Which of the following is correct sequence for ionic radius ?
Which of the following is used as “control rod” in fission reactor ?
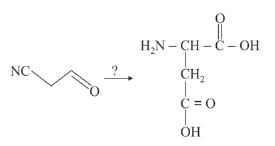
How would you accomplish the following synthesis of aspartic acid?
The Glycosidic linkages and Peptide linkages are present in:
When glucose is reduced with sodium amalgam in aqueous solution, it gives
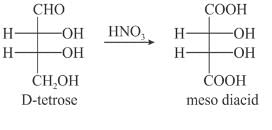
Which of the following structures is a D-aldotetrose that gives a meso diacid upon oxidation with dilute aq. HNO3?
Statement-1: A solution of sucrose in water is dextro rotatory but on hydrolysis in presence of H+ , the solution becomes leavo rotatory
Statement-2: Inversion of sugar follows first order kinetics.
Consider the following statements:
I. Bleaching action of Cl2 is due to oxidation of coloured substances
II. HOCl > HOBr > HOI : Acidic strength
III. CIF3 < BrF3 < IF3 : Polarity
Choose the correct statements.
Assertion: Nitrogen and oxygen present in the atmosphere do not react to form oxides of nitrogen.
Reason: High temperature is required for the reaction between nitrogen and oxygen.
Which of the following is a consequence of the inert pair effect?
(a) SnCl2 acts as a reducing agent.
(b) SnCl4 acts as an oxidizing agent.
(c) TlCl acts as an oxidizing agent.
(d) PbO2 is an oxidant.
Carbon and silicon belongs to 14th group. The maximum covalency of carbon in commonly occurring compounds is 4 whereas that of silicon is 6 . This is due to
Which of the following group 14 elements can decompose steam to form dioxide and liberate H2?
Baking power is more commonly used to make cakes or bread "rise". Filler in baking power is
What kind of linkage or bond exist between two monosaccharide units of lactose ?
A sample containing 1.0 mole of an ideal gas is expanded reversibly and isothermally to ten times its original volume in two separate experiments. The expansion is carried out at 300 K and 600 K, respectively. Choose the correct option.
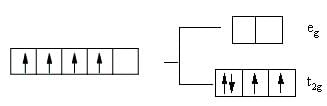
In the complex ion 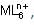 M has four d-electrons and L is a strong field ligand. According to crystal field theory, the magnetic properties of the complex ion correspond to how many unpaired electrons?
M has four d-electrons and L is a strong field ligand. According to crystal field theory, the magnetic properties of the complex ion correspond to how many unpaired electrons?
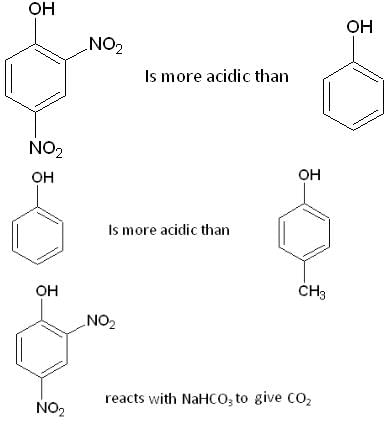
Which one of the following compounds is most likely to give effervescence of CO2 with NaHCO3?
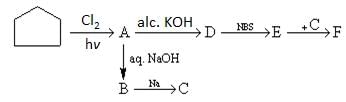
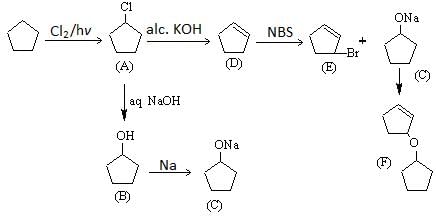
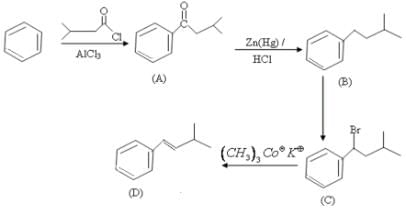
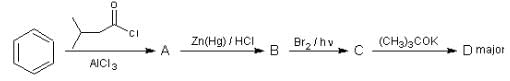
The major product (D) in the following reaction sequence is:
The difference in vibrational kinetic energies per mole of CO2 and SO2 at temperature T, according to law of equipartition of energy, is equal to