Test: Periodic Table - BMAT MCQ
10 Questions MCQ Test - Test: Periodic Table
Which of the following does NOT depend on the attraction of the bonding pair towards the nucleus?
Which of the following statements accurately describes the different classifications of elements?
Which of the following statements most accurately describes the characteristics of the transition metals?
Which of the following inequalities accurately describes the relationship between the two ionic species in terms of size?
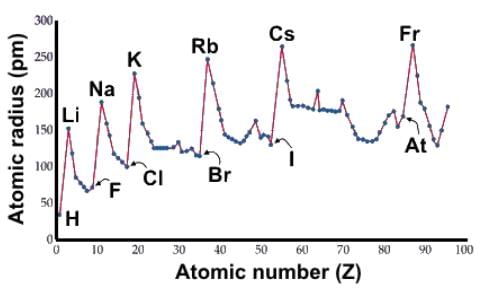
Which of the following statements about atomic radii accurately describes the data below?
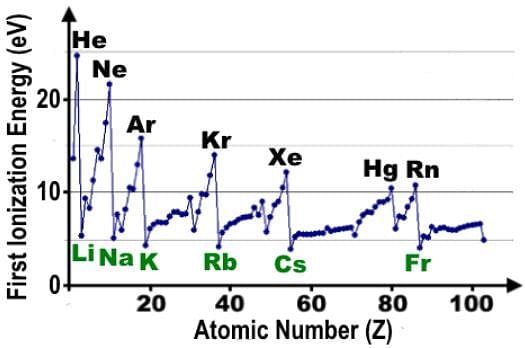
Which of the following statements accurately describes the first ionization energy data shown in the table?
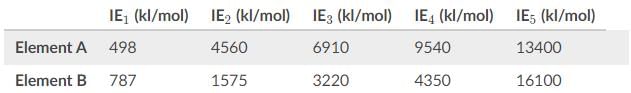
Based on the values for the first through fifth ionization energies in the table, what is the most likely identity of Element A and B?
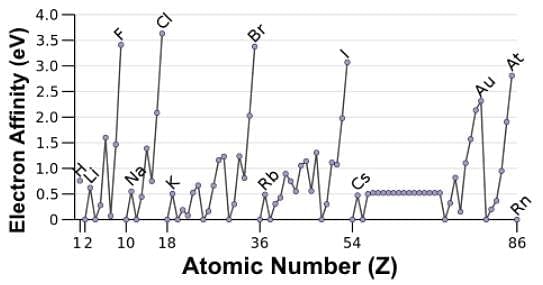
Which of the following statements accurately describes the electron affinity data in the chart below?
Which of the following statements describes a correct step in the formation of an ionic bond between sodium and chlorine?
Which of the following statements does NOT accurately describe the atomic trends?



















